Genome maintenance and variability: enzymology of DNA replication and repair
Research summary:
Work done in the last decade has revealed that DNA replication is a major source of genomic instability, due to rearrangements that occur at sites of stalled replication forks. These problems are overall defined as “replication stress” (RS). Along the last five years we have contributed to demostrate that the majority of DNA lesions or blocking structures occurring in the template DNA may be “tolerated” during RS, mainly through the generation of PrimPol-mediated new replicative starting points (namely primers) in the leading strand, immediately beyond the lesion. Importantly, our discovery of PrimPol leads to reconsider the molecular scenario in which the DNA lesion is subsequently repaired, implying the existence of remaining small DNA gaps containing the lesion, generated after PrimPol-mediated restart, that need to be filled, processed, and finally sealed. We are currently interested in the identification and characterization of potential PrimPol interactors, and in the basic mechanism of the subsequent damage tolerance and repair transactions needed behind, after PrimPol-mediated fork restart.
It is worth mentioning that PrimPol inactivation or removal during the replication of nuclear DNA entails a significant reduction of replication fork speed, indicating its relevant role in physiological conditions. Thus, whereas PrimPol-deficient mice and siRNA-depleted human cells were viable, they show large symptoms of genomic instability, very likely related to the augmented DNA RS in these cells.
On the other hand, in the presence of RS induced by replication inhibitors or topoisomerase poisons, such as camptothecin and etoposide, PrimPol deficiency generates a strong and persistent increase in RS signaling, leading to augmented genetic instability, a hallmark of cancer cells. Altogether, our data strongly suggest that PrimPol is a novel and relevant target in cancer. Its deficiency, either due to specific inactivating mutations or to the use of specific inhibitors, in combination with other well known genotoxic agents, would lead to high replicative stress burden causing tumor cells death by replication catastrophe. Thus, another objective of our research is the analysis of human PrimPol regulation, and the characterization of human PrimPol cancer variants with altered specific activity and primase deficiency, that could have a pronostic value in cancer.
From a biotechnological perspective, we have developed a high-trhougput secreening to obtain PrimPol variants with altered specificity that could be relevant for a novel PrimPol-based DNA amplification method named TruePrime™, currently commercialized by Expedeon AG (currently 4BaseBio AG), for the diagnosis of cancer in liquid biopsies.
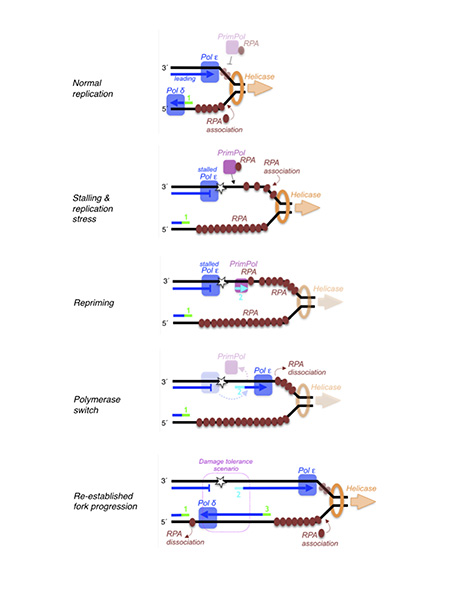
Figure 1. Schematic represention of PrimPol acting at a stalled replication fork. After PrimPol recruitment via RPA and subsequent synthesis of a DNA primer, fork progression is re-stablished, and a lesion-containing gap is left behind, which requires the direct action of TLS polymerases, or template-switch (damage avoidance) events to copy the newly synthesized/undamaged daughter strand. RNA primers in the lagging strand (green) or an eventual DNA primer made by PrimPol in the leading strand (cyan) are numbered according to their proposed order of synthesis.
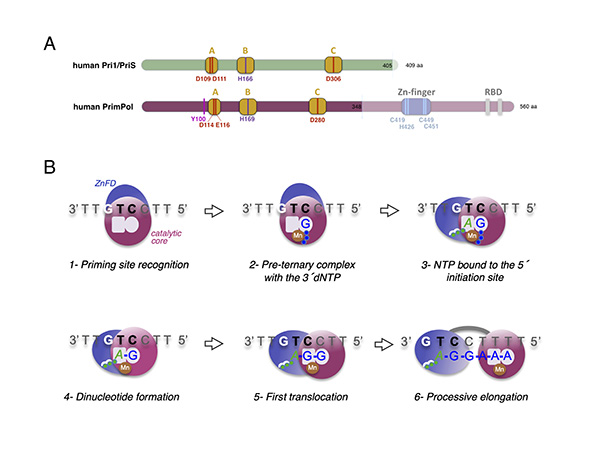
Figure 2. Mechanism of DNA priming by human PrimPol. A) Structural comparison of human Pri1/PriS/p49 and human PrimPol. B) Sequential steps during primer synthesis by human PrimPol.

| Last name | Name | Laboratory | Ext.* | Professional category | |
|---|---|---|---|---|---|
| Blanco Dávila | Luis | 403 | 4685 | lblanco(at)cbm.csic.es | E. Profesores de Investigación de Organismos Públicos de Investigación |
| Guerra González | Susana | 403 | 4686 | sguerra(at)cbm.csic.es | Tco.Sup.Activ.Técn.y Profes. GP3 |
| Jiménez Juliana | Marcos | 403 | 4686 | marcos.j(at)cbm.csic.es | Titulado Sup. Actividades Tecn. y Prof.GP1 |
| Jiménez Mejías | Alejandra | 403 | 4686 | Estudiante TFM | |
| Martínez Jiménez | María Isabel | 403 | 4686 | maria_m(at)cbm.csic.es | Doctor FC3 |
| Olmos Guevara | Regina | 403 | 4686 | Estudiante |
Relevant publications:
- Blanco L, Calvo PA, Diaz-Talavera A, Carvalho G, Calero N, Martínez-Carrón A, Velázquez-Ruiz C, Villadangos S, Guerra S, Martínez-Jiménez MI (2019) Mechanismn of DNA primer synthesis by human PrimPol. Enzymes 45, 289-310. Review.
- Calvo PA, Sastre-Moreno G, Perpiñá C, Guerra S, Martínez-Jiménez MI, Blanco L (2019) The invariant glutamate of human PrimPol DxE motif is critical for its Mn2+-dependent distinctive activities. DNA Repair (Amst) 77, 65-75.
- Díaz-Talavera A, Calvo PA, González-Acosta D, Díaz M, Sastre-Moreno G, Blanco-Franco L, Guerra S, Martínez-Jiménez MI, Méndez J, Blanco L (2019) A cancer-associated point mutation disables the steric gate of human PrimPol. Rep. Feb 4;9(1):1121.
- Martínez-Jiménez MI, Calvo PA, García-Gómez S, Guerra-González S, Blanco L (2018) The Zn-finger of human PrimPol is required to stabilize the initiating nucleotide during DNA priming. Nucleic Acids Res. 46, 4138-4151.
- Torregrosa-Muñumer R, Forslund JME, Goffart S, Pfeiffer A, Stojkovič G, Carvalho G, Al-Furoukh N, Blanco L, Wanrooij S, Pohjoismäki JLO. (2017) PrimPol is required for replication reinitiation after mtDNA damage. Proc Natl Acad Sci U S A. 114, 11398-11403.
- Agudo R, Calvo PA, Martínez-Jiménez MI, Blanco L (2017) Engineering human PrimPol into an efficient RNA-dependent-DNA primase/polymerase. Nucleic Acids Res. 45, 9046-9058.
- Martínez-Jiménez MI, Lahera A, Blanco L (2017) Human PrimPol activity is enhanced by RPA. Sci Reports. 7(1):783. doi: 10.1038/s41598-017-00958-3. PMID: 28396594.
- Picher AJ, Wafzig O, Krüger C, García-Gómez S, Martínez-Jiménez MI, Díaz-Talavera A, Blanco L* and Armin Schneider S* (2016) TruePrime, a novel method for whole genome amplification from single cells based on TthPrimPol. *co-corresponding authors. Nature Commun. 7:13296 | doi: 10.1038/ncomms13296.
- Mouron S, Rodriguez-Acebes S, Martinez-Jimenez MI, Garcia-Gomez S, Chocrón ES, Blanco L*, Mendez J* (2013). Repriming of DNA synthesis at stalled replication forks by human PrimPol. *co-corresponding authors. Nature Structural & Molecular Biology 20, 1383-1389.
- Garcia-Gomez S, Reyes A, Martinez-Jimenez MI, Chocrón ES, Mouron S, Terrados G, Powell C, Salido, E, Mendez J, Holt IJ, Blanco L (2013). PrimPol, an Archaic Primase/Polymerase Operating in Human Cells. Molecular Cell 52, 541-53.
Doctoral theses:
- Sara García Gómez (2013). “PrimPol, una nueva primasa/polimerasa en células humanas”. Universidad Autónoma de Madrid. Supervisors: Luis Blanco Dávila & María I. Martínez Jiménez.
- Ana Gómez Bedoya (2013). “Análisis estructura-función de la DNA polimerasa lambda humana y su implicación en la reparación del DNA mediante NHEJ”. Universidad Autónoma de Madrid. Supervisor: Luis Blanco Dávila.
- Ana Aza Montoya (2014). “In vivo role of DNA polymerases lambda and mu in Genome Stability”. Universidad Autónoma de Madrid. Supervisors: Luis Blanco Dávila & Gloria Terrados Aguado.
- Guillermo Sastre Moreno (2016). “Polymerases specialized in damage tolerance and DNA double-strand break repair”. Universidad Autónoma de Madrid. Supervisors: Luis Blanco Dávila & José F. Ruiz Pérez.
- Patricia A. Calvo Fernández (2019). “Structure-function analysis of human PrimPol”. Universidad Autónoma de Madrid. Supervisors: Luis Blanco Dávila & María I. Martínez Jiménez.
Patents:
- A. Picher y L. Blanco. Title: Methods for amplification and sequencing using thermostable TthPrimPol. Priority number: EP13159629. Country of priority: Europe Priority date: 15 March 2013. Owner: Expedeon AG.
Other activities:
- Coordinator of the module (BM6): "Estabilidad de la Información Genética: Replicación, Reparación y Mutagénesis", Master of Molecular and Celular Biology, Universidad Autónoma de Madrid (UAM).
- XX Carmen & Severo Ochoa Award in Molecular Biology (2014).




















