Cellular plasticity in development and cancer
Research summary:
The goal of our research group is to understand how cellular identity is determined, and how it becomes deregulated in pathological conditions, such as cancer and congenital syndromes. We focus our research on the development and diseases of the immune system and, as experimental tools, we use: 1) genetically engineered mouse models (GEMMs) in which we modify the expression of transcription factors and epigenetic regulators, either normal or oncogenic, and 2) samples from patients with genetic syndromes.
CELLULAR PLASTICITY IN THE ORIGIN OF HUMAN CANCER
In humans, in general, the oncogene responsible for the tumoral origin is present also in all the tumoral cells. However, the cancer stem cell (CSC) model postulates that cancer is a hierarchically organized tissue maintained by malignant cancer stem cells (Vicente-Dueñas et al, EMBO Journal 2013), and this suggests the possibility that the genetic information responsible for tumor generation might only be needed at the level of these cancer stem cells. We have generated mouse models of hematopoietic neoplasms like multiple myeloma, leukemias or lymphomas, and their characterization has allowed us to prove that it is indeed possible to induce tumor development in the mouse by restricting oncogene expression to the stem/progenitor cells responsible for tumor origin and maintenance (Figure 1) (Campos-Sanchez et al., Biochim. Biophys. Acta 2015). Recently, we have generated mice expressing, in their hematopoietic stem cells, the oncogenic lesion ETV6-RUNX1, responsible for the initiation of childhood B acute lymphoblastic leukemias (B-ALLs). With this model, we are investigating the role that the exposure to different agents might play in B-ALL development (Campos-Sanchez et al., Bioelectromagnetics 2019), and we have demonstrated that infections are one of the triggering factors of B-ALLs (Rodríguez-Hernández et al., Cancer Research 2017). Our results show the existence of an oncogene-initiated epigenetic reprogramming event that leads to the final development of the full-blown tumor (Vicente-Dueñas et al., Trends in Cancer 2018).
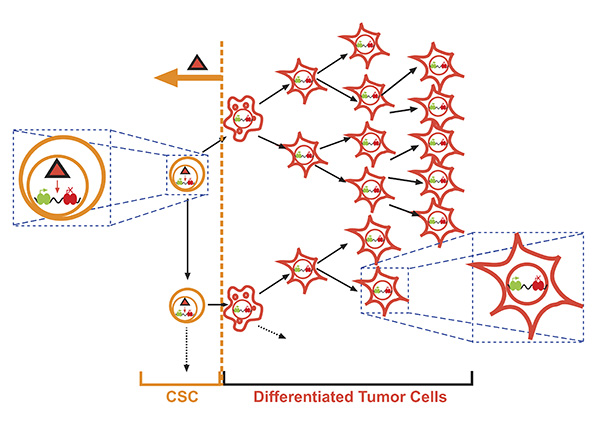
Figure 1. Generation of stem cell-driven cancer in the mouse. The expression of the oncogenic alteration is restricted to the progenitor compartment but is nevertheless capable of generating a full-blown tumour with all its differentiated cellular components. This model implicitly relies on the fact that the oncogenic presence in the cancer stem cell compartment causes (epi)genetic latent alterations [repressing (red) or activating (green)], which are responsible for the posterior appearance of the tumoral phenotype. These alterations are induced by the oncogene in the CSCs and inherited in the lower compartments where the oncogene is no longer expressed.
EPIGENETIC DEREGULATION IN HUMAN IMMUNODEFICIENCIES
Normal immune differentiation and function are not only altered in cancer, but also in many congenital syndromes of genetic origin. Wolf Hirschhorn Syndrome (WHS) is a complex, multiorganic developmental disease, and WHS patients present many severe problems, including immunodeficiencies. One of the genes affected in WHS is WHS-Candidate 1 (WHSC1), coding for a histone 3 lysine 36 methyltransferase acting as an epigenetic regulator involved in DNA damage repair and other processes (Figure 2). Furthermore, WHSC1 over-activation is implicated in several tumors, including multiple myeloma and childhood leukemias. We have characterized the immune function in Whsc1 knockout mice, and we have shown that the decreased WHSC1 function is responsible for the immunodeficiency in WHS (Figure 3) (Campos-Sánchez et al., Cell Reports 2017). Therefore, we have proposed (Martínez-Cano et al., Frontiers in Cell. Dev. Biol. 2019) that WHS is a representative example of an emerging type of immunodeficiencies in which alterations in epigenetic regulation are at the root of the disease (Figure 4), and we have an ideal preclinical model for the testing of new potential therapeutic approaches aimed at improving the patient’s conditions (Campos-Sánchez et al., Trends in Immunology 2019). Gaining insight into the molecular biology of WHS-associated immunodeficiency can help us to better understand the role of epigenetics in normal and pathological hematopoietic development and to develop future potential therapeutic interventions for them.
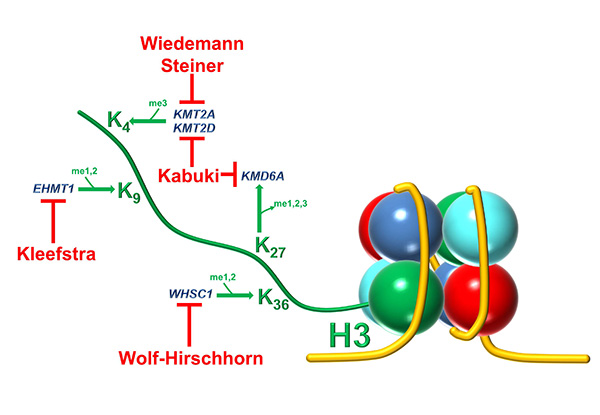
Figure 2. Histone methylation alterations in human primary immunodeficiencies. The enzymes responsible for methylation or demethylation of the indicated Lysine (K) residues are shown in blue, and the syndromes in which these enzymes are defective are indicated in red.
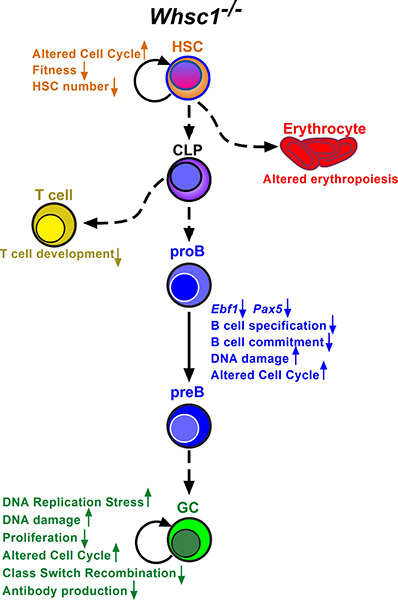
Figure 3. Hematopoietic defects in the absence of Whsc1, organized around the impairment in B-cell development and function.
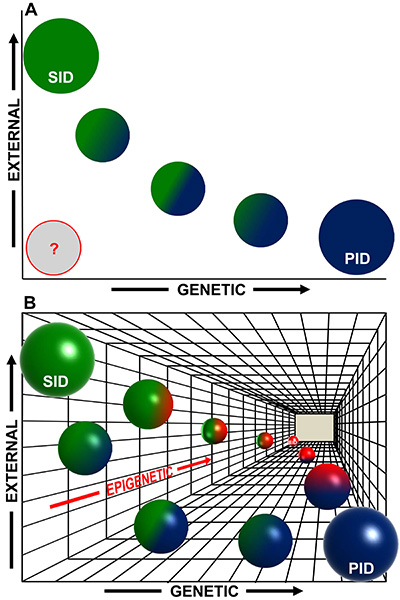
Figure 4. An epigenetic perspective on the origin of human immunodeficiencies (IDs). (A) Traditionally, two main variables have been considered for the major subdivision of human IDs: primary IDs (PIDs), initiated by genetic causes (“x”-axis) or secondary IDs (SIDs), triggered by external exposures (“y”-axis). Therefore, strict PIDs, (blue circle) would be at the rightmost end of the x-axis and purely SIDs, (green circle) would be at the upper end of the y-axis. There is also a continuous gradient of intermediate cases with a mixed, variable, contribution of genetic and environmental causes. Additionally, in many cases, the etiology of IDs is still uncertain (represented by a gray circle). (B) Now we have evidences showing that epigenetics constitutes a third underlying “z”-axis (shown in gradations of red) complementary and subjacent to the previous ones. In both PIDs and SIDs, epigenetics can play a role in the initiation or the manifestation of the disease, with a degree of contribution that can vary among diseases (green–red and blue–red spheres). The most extreme case would be that of IDs triggered only by epigenetic alterations (Epigenetic ID, EID, red sphere at the end of the z-axis). In any case, also in this 3D scheme, the spheres represent points in what most likely is a continuum landscape of possible combinations contributing to the development of human IDs.

| Last name | Name | Laboratory | Ext.* | Professional category | |
|---|---|---|---|---|---|
| Bailey Maldonado | Rodrigo | 325 | 4469 | rbailey(at)cbm.csic.es | Ayudante Investigación |
| Cobaleda Hernández | César | 325 | 4692 | cesar.cobaleda(at)csic.es | E. Investigadores Científicos de Organismos Públicos |
| Ferreiro García | Nerea | 325 | 4469/4692 | Estudiante | |
| Martínez Cano | Jorge | 325 | 4469 | jorge.martinez(at)cbm.csic.es | Investigador Indef. GP1 |
Other activities:
- National Award for Research in the field of Rare Diseases 2015, awarded by the Spanish Federation of Rare Diseases (FEDER).
- “Ad hoc” member of the committee of the German Federal Office for Radiation Protection (BfS), in charge of defining future research programs on the role of environmental factors in the pathogenesis of childhood leukemia.




















