Modulation of antiviral immunity by viral proteases and noncoding RNAs
Research summary:
Our research interest is focused on i) the interplay between Foot-and-mouth disease virus (FMDV) and the host innate immunity system, including the characterization of proteins involved in detection of the viral RNA genome by cellular immune sensors, as well as the characterization of the immune evasion mechanisms exerted by the virus to counteract the host antiviral response based on triggering of the type-I interferon pathway in infected cells. The outcome of the balance between the antiviral response and viral antagonism may determine the onset of disease and pathogenesis and we are actively working on the identification of host innate effector proteins which are targets for the viral proteases. We have recently reported a novel mechanism of viral evasion based on the cleavage of innate sensor LGP2 by the FMDV Leader protease. The proteolytic activity of the two virally encoded proteases will be tested for interference with different signalling routes known to respond to infection by RNA viruses (RIG-I-like receptors, Toll-like receptors, cGAS/STING); ii) the biotherapeutic applications of synthetic non-coding RNAs derived from the viral genome, known to elicit a broad spectrum antiviral activity. We have shown that RNA delivery enhanced the specific B- and T-cell mediated immune responses elicited after vaccination, increasing the rate of protection against viral infection. The use of these molecules as vaccine adjuvants and their putative broad-spectrum antimicrobial activity are currently being addressed. The results derived from these studies will contribute to gain knowledge on the interaction between viral pathogens and the innate immune system of the host cell and to design new and more effective strategies against RNA viruses and likely other infectious diseases. Also, learning from how viruses counteract host immune responses through specific targeting of relevant effector proteins may help in the design of new therapeutic strategies against type I interferonopathies.
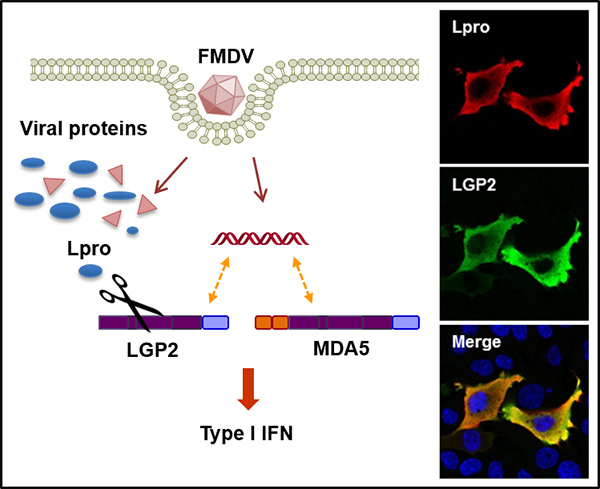
Fig. 1.

| Last name | Name | Laboratory | Ext.* | Professional category | |
|---|---|---|---|---|---|
| Polo Hernández | Miryam | 105 | 4729 | mpolo(at)cbm.csic.es | Titulado Sup. Actividades Tecn. y Prof.GP1 |
| Sáiz Zalabardo | Margarita | 105 | 4729 | msaiz(at)cbm.csic.es | E.Científicos Titulares de Organismos Públicos de Investigación |
Relevant publications:
- Miguel Rodríguez Pulido (Researcher ID L-7644-2014). Tit Rodriguez Pulido, M., Sanchez-Aparicio, M.T., Martinez-Salas, E., Garcia-Sastre, A., Sobrino, F., and Saiz, M. (2018). Innate immune sensor LGP2 is cleaved by the Leader protease of foot-and-mouth disease virus. PLoS Pathog 14(6), e1007135.
- Rodriguez Pulido, M., Del Amo, L., Sobrino, F., and Saiz, M. (2018). Synthetic RNA derived from the foot-and-mouth disease virus genome elicits antiviral responses in bovine and porcine cells through IRF3 activation. Vet Microbiol 221, 8-12.
- Borrego, B., Blanco, E., Rodriguez Pulido, M., Mateos, F., Lorenzo, G., Cardillo, S., Smitsaart, E., Sobrino, F, and Saiz, M. (2017). Combined administration of synthetic RNA and a conventional vaccine improves immune responses and protection against foot-and-mouth disease virus in swine. Antiviral Res 142, 30-36.
- Rodriguez Pulido, M., and Saiz, M. (2017). Molecular Mechanisms of Foot-and-Mouth Disease Virus Targeting the Host Antiviral Response. Front Cell Infect Microbiol 7, 252. doi: 10.3389/fcimb.2017.00252.
- Borrego, B., Rodriguez-Pulido, M., Revilla, C., Alvarez, B., Sobrino, F., Dominguez, J., and Saiz, M. (2015). Synthetic RNAs Mimicking Structural Domains in the Foot-and-Mouth Disease Virus Genome Elicit a Broad Innate Immune Response in Porcine Cells Triggered by RIG-I and TLR Activation. Viruses 7(7), 3954-3973. doi: 10.3390/v7072807.
- Borrego, B., Rodriguez-Pulido, M., Mateos, F., de la Losa, N., Sobrino, F., and Saiz, M. (2013). Delivery of synthetic RNA can enhance the immunogenicity of vaccines against foot-and-mouth disease virus (FMDV) in mice. Vaccine 31(40), 4375-4381.
- Rodriguez-Pulido, M., Borrego, B., Sobrino, F., and Saiz, M. (2011). RNA structural domains in non-coding regions of foot-and-mouth disease virus genome trigger innate immunity in porcine cells and mice. J Virol 85(13), 6492-6501.
- Rodriguez-Pulido, M., Sobrino, F., Borrego, B., and Saiz, M. (2011). Inoculation of newborn mice with non-coding regions of foot-and-mouth disease virus RNA can induce a rapid, solid and wide-range protection against viral infection. Antiviral Res 92(3), 500-504.
- Pulido, M.R., Sobrino, F., Borrego, B., and Saiz, M. (2010). RNA immunization can protect mice against foot-and-mouth disease virus. Antiviral Res 85(3), 556-558.
- Rodriguez Pulido, M., Sobrino, F., Borrego, B., and Saiz, M. (2009). Attenuated foot-and-mouth disease virus RNA carrying a deletion in the 3' noncoding region can elicit immunity in swine. J Virol 83(8), 3475-3485.
Doctoral theses:
- Miguel Rodríguez Pulido (Researcher ID L-7644-2014). Título: “Estructura y función de la región 3´ no codificante del virus de la fiebre aftosa. Aplicación a nuevas estrategias vacunales basadas en RNA”. Universidad Autónoma de Madrid. Facultad de Ciencias. Departamento de Biología Molecular (03/07/2009).
Patents:
- M. Sáiz, F. Sobrino, B. Borrego, M. Rodriguez, J.C. Sáiz y M.A Martín. Uso de una región no codificante del genoma del virus de la fiebre aftosa para la elaboración de un medicamento antiviral. ES 2401899 B1; P 201130445 (8); C12N 15/11 (2006.01); C12N 15/117 (2010.01) CSIC (50%)-INIA-CReSA.PCT and Argentina.




















