Signaling pathways in mouse models of metabolic and cardiovascular pathologies
Research summary:
Our group has been working for the past decade on analyzing some of the cellular processes and intracellular signal transduction pathways that control and regulate the response of an organism to physiological and pathological processes involving metabolic rewiring. We have focused, in particular, on the integration that occurs among different organs during obesity, insulin resistance and cardiovascular disease conditions.
Using diets to model human disease, our group has studied the response of several metabollicaly-relevant organs and tissues as the heart, adipose tissue or the liver to pathological insults such as nutrient overload. We have also investigated the molecular events controlling the adaptation of the body to physiological responses such as fasting and calorie restriction.
We are paying particular attention to the molecular determinants that are key to how aged animals control these physiopathological responses in comparison to young mice. Specifically, we analyze molecular and cellular events that may differ between aged and young animals in relationship with metabolic responses such as glucose handling, adiposity, vascular function and cardiac or hepatic steatosis among others.
Finally, we have recently initiated a research line aimed at deciphering some of the tissue, cell and molecular aspects that differentiate female and male responses to nutrient overload, insulin resistance, obesity and cardiovascular health.
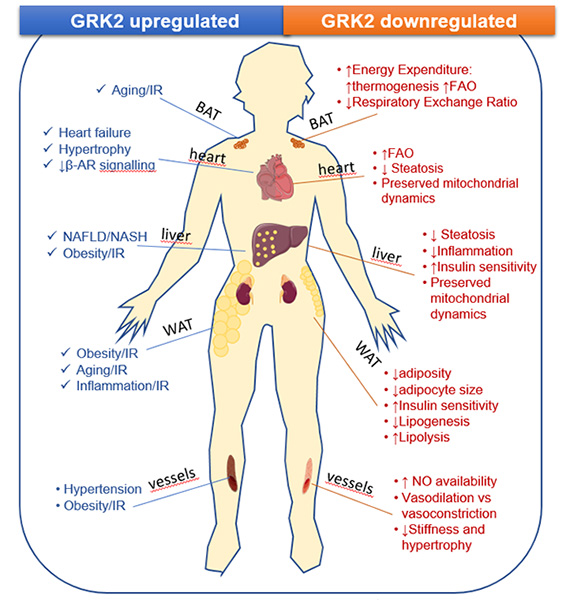
Graphical summary of recent contributions of our group regarding the influence of signaling cascades in the regulation of cellular and tissue processes involved in human pathophysiology (mostly performed in animal models of human disease). IR: insulin resistance; FAO, fatty acid oxidation; BAR, beta adrenergic receptor; NAFLD, non alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NO nitric oxide.

| Last name | Name | Laboratory | Ext.* | Professional category | |
|---|---|---|---|---|---|
| Murga Montesinos | Cristina | 320/114.2 | 4641 | cmurga(at)cbm.csic.es | Catedrático Universidad, GA |
| Portilla Tundidor | Yadileiny | 320 | 4652 | yportilla(at)cbm.csic.es | Titulado Sup.de Actividades Técn. y Profes. GP1 |
| Vida Rueda | Mª Carmen | 320 | 4652 | mcvida(at)cbm.csic.es | Investigador Doctor |
Relevant publications:
- Arcones AC, Vila-Bedmar R, Mirasierra M, Cruces-Sande M, Vallejo M, Jones B, Tomas A, Mayor F Jr, Murga C. GRK2 regulates GLP-1R-mediated early phase insulin secretion in vivo. BMC Biology 2021 19(1):40. doi: 10.1186/s12915-021-00966-w. IF: 6.765 (Q1)
- Arcones AC, Martínez-Cignoni MR, Vila-Bedmar R, Yáñez C, Lladó I, Proenza AM, Mayor F Jr, Murga C. Cardiac GRK2 Protein Levels Show Sexual Dimorphism during Aging and Are Regulated by Ovarian Hormones. Cells 2021 10(3):673. doi: 10.3390/cells10030673. IF : 4,366 (Q1)
- Arcones AC, Murga C, Penela P, Inserte J and Mayor F Jr*. G protein–coupled receptor kinase 2 at crossroads of metabolic and cardiovascular diseases. Current Op Endo Metabol Research (2021) 16:75-85 doi:10.1016/j.coemr.2020.09.004
- González-Amor M, Vila-Bedmar R, Rodrigues-Díez R, Moreno-Carriles R, Arcones AC, Cruces-Sande M, Salaices M, Mayor F Jr, Briones AM, Murga C. Myeloid GRK2 Regulates Obesity-Induced Endothelial Dysfunction by Modulating Inflammatory Responses in Perivascular Adipose Tissue. Antioxidants (Basel). 2020 Oct 4;9(10):953. doi: 10.3390/antiox9100953. IF: 5,0 (Q1)
- Vila-Bedmar R, Cruces-Sande M, C. Arcones A, Willemen HLDM, Prieto P, Moreno-Indias I, Díaz-Rodríguez D, Francisco S, I. Jaén RI, Gutiérrez-RepisoC, HeijnenCJ, Boscá L, Fresno M, Kavelaars A, Mayor Jr F and Murga C*. GRK2 levels in myeloid cells modulate adipose-liver crosstalk in high fat diet-induced obesity. Cellular and Molecular Life Sciences (CMLS) 2020;77(23):4957-4976 IF: 7 (Q1). doi: 10.1007/s00018-019-03442-5
- Marta Cruces-Sande, Alba C. Arcones, Rocío Vila-Bedmar, Almudena Val-Blasco, Kfir Sharabi, Daniel Díaz-Rodríguez, Pere Puigserver, Federico Mayor Jr., Cristina Murga. Autophagy mediates hepatic GRK2 degradation to facilitate glucagon-induced metabolic adaptation to fasting. The FASEB Journal. 2019;00:1–11 doi: 10.1096/fj.201901444R
- Arcones AC, Cruces-Sande M, Ramos P, Mayor F Jr, Murga C. Sex Differences in High Fat Diet-Induced Metabolic Alterations Correlate with Changes in the Modulation of GRK2 Levels. Cells. 2019 Nov 19;8(11). pii: E1464. doi: 10.3390/cells8111464.
- Murga C, Arcones AC, Cruces-Sande M, Briones AM, Salaices M, Mayor F Jr. G Protein-Coupled Receptor Kinase 2 (GRK2) as a Potential Therapeutic Target in Cardiovascular and Metabolic Diseases. Front Pharmacol. 2019 Feb 19;10:112. doi: 10.3389/fphar.2019.00112.
- Cruces-Sande M, Vila-Bedmar R, Arcones AC, González-Rodríguez Á, Rada P, Gutiérrez-de-Juan V, Vargas-Castrillón J, Iruzubieta P, Sánchez-González C, Formentini L, Crespo J, García-Monzón C, Martínez-Chantar ML, Valverde ÁM, Mayor F Jr, Murga C. Involvement of G protein-coupled receptor kinase 2 (GRK2) in the development of non-alcoholic steatosis and steatohepatitis in mice and humans. Biochim Biophys Acta Mol Basis Dis. 2018 Dec;1864(12):3655-3667. doi: 10.1016/j.bbadis.2018.09.027.
- Mayor F Jr, Cruces-Sande M, Arcones AC, Vila-Bedmar R, Briones AM, Salaices M, Murga C. G protein-coupled receptor kinase 2 (GRK2) as an integrative signalling node in the regulation of cardiovascular function and metabolic homeostasis. Cell Signal. 2018 Jan;41:25-32. doi: 10.1016/j.cellsig.2017.04.002.
- Lucas E, Vila-Bedmar R, Arcones AC, Cruces-Sande M, Cachofeiro V, Mayor F Jr, Murga C. Obesity-induced cardiac lipid accumulation in adult mice is modulated by G protein-coupled receptor kinase 2 levels. Cardiovasc Diabetol. 2016 Nov 10;15(1):155.
- Vila-Bedmar R, Cruces-Sande M, Lucas E, Willemen HL, Heijnen CJ, Kavelaars A, Mayor F Jr, Murga C. Reversal of diet-induced obesity and insulin resistance by inducible genetic ablation of GRK2. Sci Signal. 2015 Jul 21;8(386):ra73. doi: 10.1126/scisignal.aaa4374
- Lucas E, Jurado-Pueyo M, Fortuño MA, Fernández-Veledo S, Vila-Bedmar R, Jiménez-Borreguero LJ, Lazcano JJ, Gao E, Gómez-Ambrosi J, Frühbeck G, Koch WJ, Díez J, Mayor F Jr, Murga C. Downregulation of G protein-coupled receptor kinase 2 levels enhances cardiac insulin sensitivity and switches on cardioprotective gene expression patterns. Biochim Biophys Acta. 2014 Dec;1842(12 Pt A):2448-56. doi: 10.1016/j.bbadis.2014.09.004.
- Willemen HL, Campos PM, Lucas E, Morreale A, Gil-Redondo R, Agut J, González FV, Ramos P, Heijnen C, Mayor F Jr, Kavelaars A, Murga C. A novel p38 MAPK docking-groove-targeted compound is a potent inhibitor of inflammatory hyperalgesia. Biochem J. 2014 May 1;459(3):427-39. doi: 10.1042/BJ20130172.
- Avendaño MS, Lucas E, Jurado-Pueyo M, Martínez-Revelles S, Vila-Bedmar R, Mayor F Jr, Salaices M, Briones AM, Murga C. Increased nitric oxide bioavailability in adult GRK2 hemizygous mice protects against angiotensin II-induced hypertension. Hypertension. 2014 Feb;63(2):369-75. doi: 10.1161/HYPERTENSIONAHA.113.01991
Doctoral theses:
- Alba Concepción Arcones. "Influence of GRK2 levels in the modulation of glucose homeostasis in health and disease". Facultad de Ciencias. Universidad Autónoma de Madrid. 2021 (Mención Internacional)
- Marta Cruces Sande. “Influence of GRK2 levels in hepatic pathophysiology”. Facultad de Ciencias. Universidad Autónoma de Madrid. 27 de abril de 2018
- Pedro M. Campos Muelas. “Identificacion de peptidos y farmacos inhibidores basados en un nuevo mecanismo de inactivación descubierto para P38 MAPK”. Facultad de Ciencias. Universidad Autónoma de Madrid. 20 de enero de 2016
- Elisa Lucas Fernández. “GRK2 in the cardiovascular disease continuum: role in hypertension and insulin resistance”. Facultad de Ciencias. Universidad Autónoma de Madrid. 9 de abril de 2015
- María Jurado Pueyo. “Nuevas funciones celulares de GRKs: Implicaciones fisio-patológicas”. Facultad de Ciencias. Universidad Autónoma de Madrid. 21 de junio de 2010
- Sandra Peregrín Pedrique. “Interactoma de GRKs: nuevas relaciones funcionales con MAPKs”. Facultad de Ciencias. Universidad Autónoma de Madrid. 20 de Julio de 2005
Patents:
- PCT/EP2006/005542 (2010): New phosphorylation site of mitogen-activated protein kinases, modified proteins and applications
- PCT /ES2011/070774 (2013): p38 inhibitor peptide and uses thereof
- PCT/ES2012/070762 (2013): Drugs for inhibiting p38 and uses thereof




















