Pathogenic mechanisms of Alzheimer’s disease
Research summary:
To identify genes and mechanisms involved in the neurodegeneration characteristic of Alzheimer's disease (AD), we developed cell models that reflect different aspects of the pathogenesis of the disease. These models allow us to identify new genes/functions associated with AD, which could be therapeutic targets for the disease. To do this, we analyze differential gene expression in the models and develop genetic association studies and biomarkers in clinical samples. In recent years, we have focused on models of herpes simplex virus 1 (HSV-1) infection that present characteristic markers of AD, including alterations in the trafficking, metabolism and proteolysis of the amyloid precursor protein (APP), as well as in the phosphorylation of the tau protein.
The study of the lysosome autophagy pathway in these models indicates that HSV-1, especially in the presence of oxidative stress, profoundly affects the final stages of the pathway, causing an increase in lysosomal content accompanied by a significant reduction in the activity of different cathepsins and in the degradation of lysosomal substrates. The evidence that another alpha-herpesvirus with neurotropic properties, HSV-2, induces the same neurodegeneration markers as HSV 1, supports the possible involvement of various infectious agents like as herpesviruses in AD-associated neurodegeneration.
The results of the functional and genetic/biomarker association studies developed so far support the hypothesis that lysosomal function failure could constitute a relevant mechanism in HSV-1-induced neurodegeneration and, in general, in AD type neurodegeneration, so our group is focused on the study of this functional pathway. Currently, we are developing more complex cell models, based on human NPCs, to validate our previous findings in a more physiological environment and delve deeper into the mechanisms involved.
In addition, we participate in several collaborative projects. The most numerous are those aimed to study the genetic architecture of AD, within the framework of the Spanish Consortium for Dementia Genetics (DEGESCO) and the international consortia EADB and IGAP, which continue to reveal new factors and relevant functions in the pathogenesis of this disease.
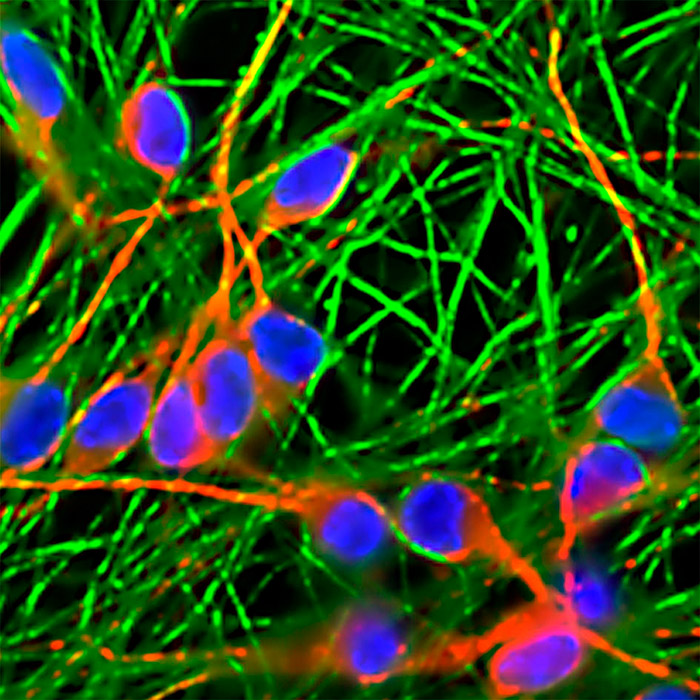
LUHMES neurons on day 7 of differentiation labeled with MAP2 (red) and B-III tubulin (green) specific antibodies. DAPI-stained nuclei are also shown.

| Last name | Name | Laboratory | Ext.* | Professional category | |
|---|---|---|---|---|---|
| Aldudo Soto | Jesús | 410 | 4674 | jaldudo(at)cbm.csic.es | Contratado Doctor CIBER |
| Beamonte Palomero | Inés | 410 | 4674 | Estudiante TFG | |
| Bullido Gómez-Heras | Mª Jesús | 410/114.2 | 4567/ 4674 | mjbullido(at)cbm.csic.es | Profesor Titular Universidad, GA |
| Martín Rico | María | 410 | 4674 | maria.martin(at)cbm.csic.es | Titulado Sup.Inv.y Laboratorio |
| Recuero Vicente | María | 410 | 4674 | mrecuero(at)cbm.csic.es | Contratado Doctor CIBER |
| Romero Solares | Alba | 410 | 4467 | Estudiante TFM | |
| Salgado Fuentes | Blanca | 410 | 4674 | bsalgado(at)cbm.csic.es | Contrato Predoctoral |
Relevant publications:
-
Herpes Simplex Virus Type 1 Induces AD-like Neurodegeneration Markers in Human Progenitor and Differentiated ReNcell VM Cells. Salgado B, Sastre I, Bullido MJ, Aldudo J. Microorganisms. 2023 May 4;11(5):1205. doi: 10.3390/microorganisms11051205. PMID: 37317179 Free PMC article.
-
Metabolic Overlap between Alzheimer's Disease and Metabolic Syndrome Identifies the PVRL2 Gene as a New Modulator of Diabetic Dyslipidemia. Guardiola M, Muntané G, Martínez I, Martorell L, Girona J, Ibarretxe D, Plana N, Bullido MJ, Vilella E, Ribalta J. Int J Mol Sci. 2023 Apr 18;24(8):7415. doi: 10.3390/ijms24087415. PMID: 37108578 Free PMC article.
-
Cholesterol dysregulation in peripheral blood mononuclear cells of Alzheimer's disease. Martín-Montes A, Recuero M, Sastre I, Vilella E, Rosich-Estragó M, Atienza M, Cantero JL, Frank-García A, Bullido MJ. J Neuroimmunol. 2022 Dec 15;373:577996. doi: 10.1016/j.jneuroim.2022.577996. Epub 2022 Oct 28. PMID: 36334319
-
New insights into the genetic etiology of Alzheimer's disease and related dementias. Bellenguez C, Küçükali F, Jansen IE, Kleineidam L, Moreno-Grau S, Amin N, Naj AC, et al Nat Genet. 2022 Apr;54(4):412-436. doi: 10.1038/s41588-022-01024-z. Epub 2022 Apr 4. PMID: 35379992 Free PMC article.
-
Matrix metalloproteinase 14 regulates HSV-1 infection in neuroblastoma cells. Llorente P, Mejías V, Sastre I, Recuero M, Aldudo J, Bullido MJ. Antiviral Res. 2021 Aug;192:105116. doi: 10.1016/j.antiviral.2021.105116. Epub 2021 Jun 6. PMID: 34107282
-
Common variants in Alzheimer's disease and risk stratification by polygenic risk scores. de Rojas I, Moreno-Grau S, Tesi N, Grenier-Boley B, Andrade V, Jansen IE, Pedersen NL, et al Nat Commun. 2021 Jun 7;12(1):3417. doi: 10.1038/s41467-021-22491-8. PMID: 34099642 Free PMC article.
-
LAMP2 deficiency attenuates the neurodegeneration markers induced by HSV-1 infection. Kristen H, Sastre I, Aljama S, Fuentes M, Recuero M, Frank-García A, Martin A, Sanchez-Juan P, Lage C, Bullido MJ, Aldudo J. Neurochem Int. 2021 Jun;146:105032. doi: 10.1016/j.neuint.2021.105032. Epub 2021 Mar 27. PMID: 33781848
-
Matrix Metalloproteinase 14 Mediates APP Proteolysis and Lysosomal Alterations Induced by Oxidative Stress in Human Neuronal Cells. Llorente P, Martins S, Sastre I, Aldudo J, Recuero M, Adjaye J, Bullido MJ. Oxid Med Cell Longev. 2020 Nov 16;2020:5917187. doi: 10.1155/2020/5917187. eCollection 2020. PMID: 33282112 Free PMC article.
-
Genetic meta-analysis of diagnosed Alzheimer's disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, Boland A, et al. Nat Genet. 2019 Mar;51(3):414-430. doi: 10.1038/s41588-019-0358-2. Epub 2019 Feb 28. PMID: 30820047 Free PMC article.
-
The lysosome system is severely impaired in a cellular model of neurodegeneration induced by HSV-1 and oxidative stress. Kristen H, Sastre I, Muñoz-Galdeano T, Recuero M, Aldudo J, Bullido MJ. Neurobiol Aging. 2018 Aug;68:5-17. doi: 10.1016/j.neurobiolaging.2018.03.025. Epub 2018 Mar 29. PMID: 29689425
-
A Free Radical-Generating System Regulates Amyloid Oligomers: Involvement of Cathepsin B. Llorente P, Kristen H, Sastre I, Toledano-Zaragoza A, Aldudo J, Recuero M, Bullido MJ. J Alzheimers Dis. 2018;66(4):1397-1408. doi: 10.3233/JAD-170159. PMID: 30400084
-
Microbes and Alzheimer's Disease. Itzhaki RF, Lathe R, Balin BJ, Ball MJ, Bearer EL, Braak H, Bullido MJ, et al. J Alzheimers Dis. 2016;51(4):979-84. doi: 10.3233/JAD-160152. PMID: 26967229 Free PMC article. Review. No abstract available.
-
Herpes simplex virus type I induces an incomplete autophagic response in human neuroblastoma cells. Santana S, Bullido MJ, Recuero M, Valdivieso F, Aldudo J. J Alzheimers Dis. 2012;30(4):815-31. doi: 10.3233/JAD-2012-112000. PMID: 22475795
Awards:
- Fundación Madrid+D. Premio a las mejores patentes; accésit. (2011)
- Sociedad Madrileña de Neurología. Premio Ramón y Cajal de Investigación básica (2011)




















