Cell signalling during imaginal development in Drosophila
Research summary:
The Drosophila wing originates from an epithelial tissue that grow by proliferation during larval development and differentiate in the pupal stage into a wing and part of the thorax of the fly. Cell proliferation and differentiation are common processes in multicellular tissues, and they are directed by evolutionary conserved batteries of genes. The genetic and developmental analysis of the disc allow us to address experimentally different aspects of epithelial biology.
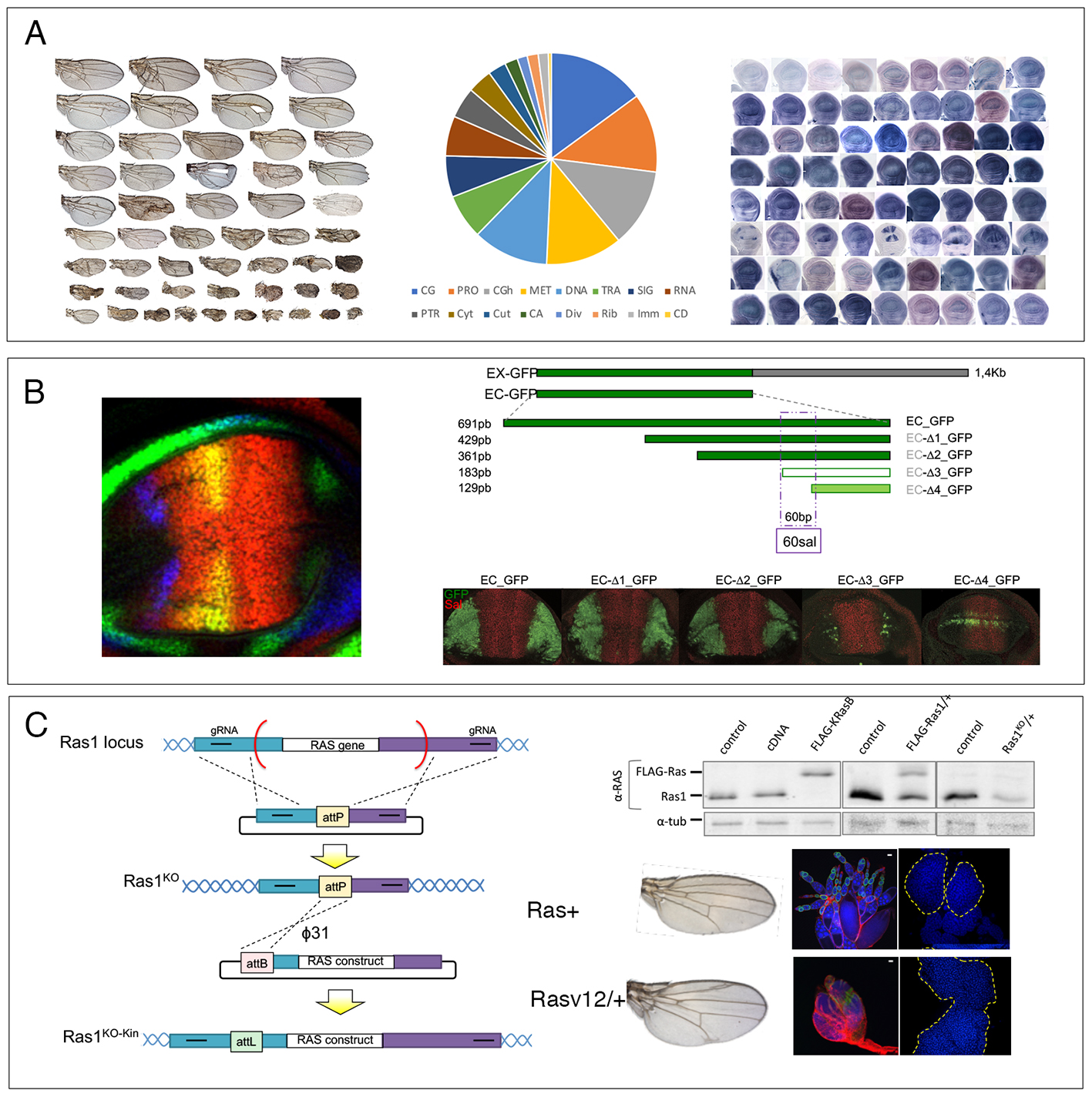
We are carrying out three research projects. The first project involves the analysis of the requirements of Drosophila genes in the wing. We grouped the 14.000 Drosophila genes into 16 functional groups (Fig. 1A) and screened UAS-RNAi lines targeting 10918 of these genes. We classified the resulting phenotypes into morphological classes affecting the size, pattern or differentiation of the wing (Fig. 1A), and correlate each mutant phenotype with the expression of the corresponding gene. Wing phenotypes reveal functional requirements, either in basic cellular functions impinging on cell viability or in wing-specific functions related to its growth and patterning, and together with gene expression patterns (Fig. 1A) constitute an optimal entry point to undertake detailed functional analysis. The second project is the analysis of the transcriptional effects of one Drosophila transcription factor (Spalt) that has a prominent role in the development of the wing disc (Fig. 1B). Spalt is a nuclear protein containing three pairs of Zn fingers and its human orthologs are involved in Towles-Brokes disease and Okihiro syndrome. We have identified a minimal DNA response element for Spalt through the analysis of the regulatory region of one of its downstream genes (Fig. 1B). Now we are defining the effects of Spalt on chromatin conformation as well as searching for Spalt co-repressors with the objective of understanding the Spalt mechanism of action. The third project concerns the Ras gene (Fig. 1C). Mutations in human Ras are common in multitude of cancers, and the Drosophila Ras gene has been used to model cancer progression in flies. Using Crisper/Cas9 and homologous recombination we have generated Drosophila transgenic lines carrying altered versions of the fly and human Ras genes (Fig. 1C). We are characterizing the consequences of activating Ras mutations when the gene is expressed at normal levels in the wing, the ovary and the lymph gland (Fig. 1C). We expect to generate genetic combinations in a background of endogenous activated Ras allowing us to model the formation and progression of tumors.

| Last name | Name | Laboratory | Ext.* | Professional category | |
|---|---|---|---|---|---|
| Celis Ibeas | José Félix de | 421 | 4673 | jfdecelis(at)cbm.csic.es | E. Profesores de Investigación de Organismos Públicos de Investigación |
| Herrera Alarcón | Sandra | 421 | 4673 | sandra.herrera(at)cbm.csic.es | M2 66,66% |
| López Varea | Ana | 421 | 4703 | alopez(at)cbm.csic.es | E. Técnicos Superiores Especializados de Organismos Públicos de Investigación |
| Pulido Vega | Diego | 421 | 4703 | dpulido(at)cbm.csic.es | E.Científicos Titulares de Organismos Públicos de Investigación |
| Ruíz Gómez | Ana | 421 | 4808 | aruiz(at)cbm.csic.es | Profesor Titular Universidad, GA |
| Sánchez de Rojas de la Osa | Paula | 421 | 4673 | Estudiante | |
| Vega Cuesta | Patricia | 421 | 4703/4808 | patricia.vega(at)cbm.csic.es | Tco. de Investigación y Laboratorio |
Relevant publications:
-
Jiménez-Martínez, M., Ostalé C.M., van der Burg, L., Galán-Martínez, J., Hardwick, J C. H., López-Pérez, R., Hawinkels, L., Stamatakis, K.and Fresno, M.. (2019) DUSP10 Is a Regulator of YAP1 Activity Promoting Cell Proliferation and Colorectal Cancer Progression. Cancers 11, 1767; doi:10.3390/cancers11111767.
-
Lawrence, P.L., Casal, J., de Celis, J. F. and Morata, G. (2019). A refutation to ‘A new A-P compartment boundary and organizer in holometabolous insect wings’. Scientific Reports 9:7049. doi: https://doi.org/10.1038/s41598-019-42668-y
-
Vega-Cuesta, P., Ruiz-Gómez, A., Molnar, C., Organista, M. F., Resnik-Docampo, M., Falo-Sanjuan, J., López-Varea, A. and de Celis, J.F. (2020). Ras2, the TC21/R-Ras2 Drosophila homologue, contributes to insulin signaling but is not required for organism viability. Dev. Biol. 461,172-183. doi: 10.1016/j.ydbio.2020.02.009
-
Casares, F., Allende, M., de Celis, J. F., Gónzalez-Reyes, A. and Martı́nez-Morales, J. R. (2020). Jose Luis Gomez-Skarmeta (1966-2020). Development 147: 1-3. doi: 10.1242/dev.197996
Doctoral theses:
- Martín Resnik Docampo (2011). La proteína MAP4K3 participa, a través de mecanismos independientes, en la regulación de las rutas de señalización Tor y JNK. Universidad Autónoma de Madrid. Director Jose F. de Celis.
- María Fernández Organista (2012). Funciones de las proteínas Spalt e identificación de sus genes diana durante el desarrollo del disco imaginal de ala de Drosophila melanogaster. Universidad Autónoma de Madrid. Director Jose F. de Celis.




















