Genetic and functional analysis of the renal filtration diaphragm in health and disease
Research summary:
Our research aims to understand the development, composition and function of the renal filtration system as well as its pathological anomalies. To this end, we make use of two model organisms, the fly Drosophila melanogaster and the zebrafish Danio rerio and apply a variety of genetic, biochemical, molecular and cell biology techniques.
Chronic kidney disease is one of the most prevalent illnesses in modern societies affecting 22% of the aged population and its incidence is increasing. It is a progressive loss of kidney function that, if untreated, becomes irreversible and leads to end-stage renal disease, requiring renal replacement therapies by dialysis or kidney transplantation. It is now accepted that chronic kidney disease is mainly triggered by insults to the glomerular podocyte, a very specialized kidney cell responsible of the initial stages of urine formation. Most of these insults disrupt slit diaphragm function. The slit diaphragm, a modified cell junction, is a multiprotein complex formed by two major structural constituents, the Immunoglobulin family members, nephrin and Neph1 and their associated intracellular partners. It acts as a molecular sieve during the process of blood ultrafiltration in the glomerulus, limiting the passage of molecules according to their size and charge. Insults to this structure induce dramatic morphological changes (foot process effacement) and lead to proteinuria.
Our recent work demonstrated a striking similarity between the vertebrate podocyte slit diaphragm and the filtration diaphragm of the fly nephrocytes that spans the functional, structural and molecular levels. These similarities allow us to exploit the power of Drosophila as a model organism to study the composition and assembly of the filtration diaphragm as well as its regulation and alterations under pathological conditions. The main objectives of our research are:
- To look for novel genes involved in the formation and function of the filtration diaphragm. To this end, we apply the powerful genetic techniques available in Drosophila. Some of the genes we have found and which are currently under study are involved in processes such as cell endocytosis, the regulation of the plasma membrane phospholipid composition or vesicular trafficking.
- To examine the dynamism of the filtration diaphragm molecular components and their regulation, by biochemistry, confocal and electron microscopy analyses. Thus, we have found that the stability of the fly filtration diaphragm is regulated by phosphorylation of Duf, the orthologue of Neph1, by the Src64B kinase. The physiological status of Duf phosphorylation impinges on the constellation of its intracellular interactors and it is necessary for diaphragm stability, whereas Duf hyperphosphorylation induces diaphragm damage.
- To study filtration diaphragm formation during development and unravel the molecular mechanisms driving its disassembly after injury and its subsequent repair. Thus, work carried out very recently by our group let us to propose a novel mechanism to account for the junctional remodelling involved in diaphragm formation during nephrocyte development and in the processes of diaphragm damage and repair. Interestingly, our studies in the zebrafish suggest that this mechanism is conserved in vertebrates. The relevance of these data resides in the fact that the morphological changes that take place in podocytes during the formation of filtration diaphragms and in response to damage and repair are driven by junctional remodelling. We are currently characterising in detail our model by analysing the fundamental steps during the process of diaphragm formation and by identifying novel components and regulators involved in junctional remodelling in nephrocytes and podocytes.
Our overarching goal is to translate to vertebrates the knowledge acquired in our experimental model, Drosophila, to help understand and treat chronic kidney disease.
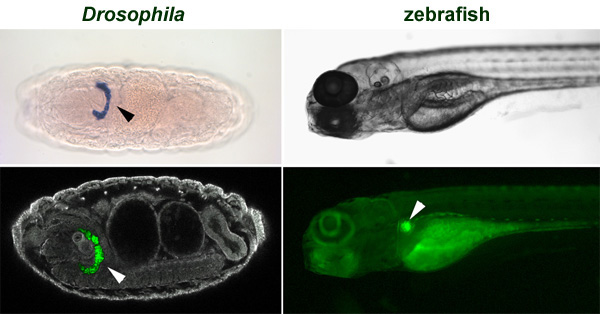
Figure 1. Our model organisms: Drosophila melanogaster and the zebrafish (Danio rerio). Drosophila embryonic nephrocytes and zebrafish pronephros are indicated by arrowheads. They are labelled by the expression of specific markers.
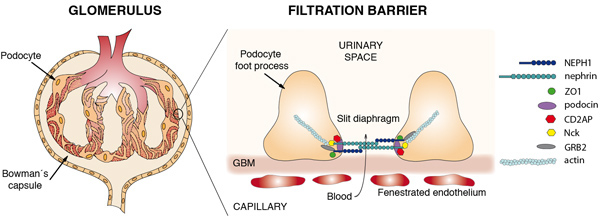
Figure 2. Schemes of the vertebrate kidney glomerulus and the glomerular filtration barrier, indicating the main protein components of the slit diaphragm.

Figure 3. Scanning electron micrograph of Drosophila larval nephrocytes (left). The ultrastructure of the mammalian slit diaphragm and Drosophila filtration diaphragm are extremely similar, as revealed by transmission electron microscopy.
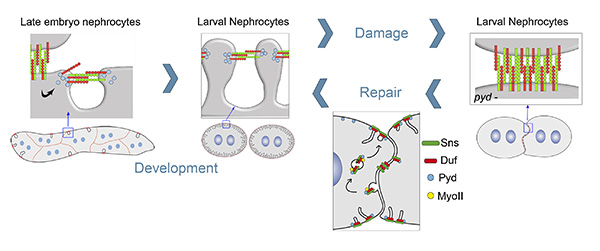
Figure 4. Scheme describing nephrocyte junctional remodelling leading to filtration diaphragm formation during development, diaphragm disassembly in response to damage and its reassembly during repair.

| Last name | Name | Laboratory | Ext.* | Professional category | |
|---|---|---|---|---|---|
| Campuzano Corrales | Sonsoles | 420.2 | 4687 | scampuzano(at)cbm.csic.es | E. Profesores de Investigación de Organismos Públicos de Investigación |
| Carrasco Rando | Marta | 420 | 4693 | mcarrasco(at)cbm.csic.es | Titulado Sup. Actividades Tecn. y Prof.GP1 |
| Culi Espigul | Joaquim | 420 | 4693 | jculi(at)cbm.csic.es | E.Científicos Titulares de Organismos Públicos de Investigación |
| Ruiz Gómez | Mar | 420.1 | 4694 | mruiz(at)cbm.csic.es | E. Investigadores Científicos de Organismos Públicos |
Relevant publications:
- M. Carrasco-Rando, J. Culi, S. Campuzano & M. Ruiz-Gómez (2023). “An acytokinetic cell division creates PIP2-enriched membrane asymmetries leading to slit diaphragm assembly in Drosophila nephrocytes”. Development 150, dev201708. doi:10.1242/dev.201708
- A. Atienza-Manuel, V. Castillo-Mancho, S. De Renzis, J. Culi and M. Ruiz-Gómez (2021). Endocytosis mediated by an atypical CUBAM complex modulates slit diaphragm dynamics in nephrocytes. Development 148, doi:10.1242/dev.199894.
- M. Carrasco-Rando, S. Prieto-Sánchez, J. Culi, A.S. Tutor and M. Ruiz-Gómez (2019). “A specific isoform of Pyd/ZO-1 mediates junctional remodeling and formation of slit diaphragms”. J. Cell Biol 218: 2294-2308. doi: 10.1083/jcb.201810171
- M. Carrasco-Rando, A. Atienza-Manuel, AS. Tutor and M. Ruiz-Gómez (2015). “Modelling renal development and disease in Drosophila”. eLS. John Wiley & Sons, Ltd: Chichester. doi: 10.1002/9780470015902.a0025981
- A.S. Tutor, S. Prieto-Sánchez and M. Ruiz-Gómez (2014). Src64B phosphorylates Dumbfounded and regulates slit diaphragm dynamics: Drosophila as a model to study nephropathies. Development 141: 367-376. doi:10.1242/dev.099408
- H. Weavers*, S. Prieto-Sánchez*, F. Grawe, A. García-López, R. Artero, M. Wilsch-Braeuninger, M. Ruiz-Gómez, H. Skaer and B. Denholm (2009). The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457: 323-326. doi: 10.1038/nature07526
Doctoral theses:
- Vicente Castillo Mancho. “Caracterización del interactoma de Scramb1, un organizador del ensamblaje del diafragma de filtración en zonas de membrana de tipo balsa lipídica”. Universidad Autónoma de Madrid. 27 de octubre de 2023, Sobresaliente “cum laude”.
- Alexandra Atienza Manuel “Función del complejo endocítico Cubilin-Amnionless y de la escramblasa se fosfolípidos Scramb1 en la biología del diafragma de filtración de Drosophila”. Universidad Autónoma de Madrid. 21 de Junio de 2019, Sobresaliente “cum laude”
- Silvia Prieto Sánchez. "Función de Dumbfounded, Sticks and stones y Polychaetoid en los nefrocitos en guirnalda de Drosophila melanogaster".Universidad Autónoma de Madrid. 15 de Junio de 2009. Sobresaliente "cum laude"
- Marta Carrasco Rando. "musculus morbidus una E3 ubiquitín ligasa de Drosophila, genera Artrina y mantiene la integridad del sarcómero". Universidad Autónoma de Madrid. 2 de Febrero de 2006. Sobresaliente "cum laude".
Awards:
- 2010 Premio a la investigación básica de la Fundación Renal Iñigo Álvarez de Toledo, por el trabajo presentado por Silvia Prieto Sánchez y Mar Ruiz Gómez, titulado: "Los nefrocitos de Drosophila como modelo de estudio de la función del diafragma de filtración"
- 2009 Premio PINP a la mejor tesis doctoral a la Dra. Silvia Prieto Sánchez




















