Molecular characterization of vascular pathogenesis and lymphomagenesis
Research summary:
To uncover novel targets for intervention in the two major causes of mortality world-wide, cardiovascular diseases and cancer, we focus on the identification of molecular mechanisms altered in these pathologies. To this end, we employ a combination of experimental approaches in vitro and in vivo, including transcriptomic and proteomic analysis, molecular and cellular biology techniques, generation of mouse models of these diseases, and advanced imaging technology.
MOLECULAR BASIS OF VASCULAR PATHOGENESIS
We are interested in uncovering novel mediators of cardiovascular pathogenesis, one of the major causes of death worldwide, because these mediators might become novel targets for therapeutic intervention. To this end, we use a combination of in vitro and in vivo experimental approaches involving transcriptomics, proteomics, cellular and molecular analysis, mouse models, and advanced imaging techniques.
Our pioneering studies, in collaboration with the group of Dr. J.M. Redondo (CNIC), show that deficiency of the metalloproteinase Adamts1 leads to thoracic aorta aneurysm (TAA) in mice due to increased Nos2-dependent NO production. We also showed that TAA was reversed using pharmacological NOS2 inhibition in mouse models, raising the possibility that blocking NO signaling could be a novel treatment for TAA. Indeed, we are pursuing clinical trials with NOS2 inhibitors in Marfan syndrome, a heritable life-threatening disease in which TAA accounts for over 90% of its mortality. These findings changed our view of the pathophysiology of TAA and prompted us to further explore the molecular and biomechanical mechanisms of TAA in collaboration with other international research groups. Much of our recent effort focuses on the identification of additional mediators of these diseases.
We also search for genes mediating pathological vascular wall remodeling, a key process in the development of hypertension and arterial diseases such as atherosclerosis and aneurysm. We had determined that calcineurin and its downstream effector Rcan1 are essential mediators of atherosclerosis, restenosis, and abdominal aorta aneurysm (AAA). Our studies allowed the identification of new pathophysiological mechanisms and new therapeutic targets in aortic diseases. Using tissue-specific inducible knockout mice, we have also uncovered a homeostatic role for Rcan1 in the aorta and that its genetic inactivation in the adult mouse predisposes to hypertension-induced intramural hematoma and subsequent AAA through mechanisms involving GSK-3b, ROCK and smooth muscle Myosin. These results prompted us to follow new exciting avenues of research into the pathogenesis of aortic diseases. In particular, we have initiated a comprehensive study aimed at identifying the role of calcineurin in the aortic transcriptome, proteome, and phosphoproteome regulated by hypertensive stimuli such as angiotensin II. We believe that these studies will identify therapeutic targets for AAA and novel therapeutic targets for arterial hypertension.
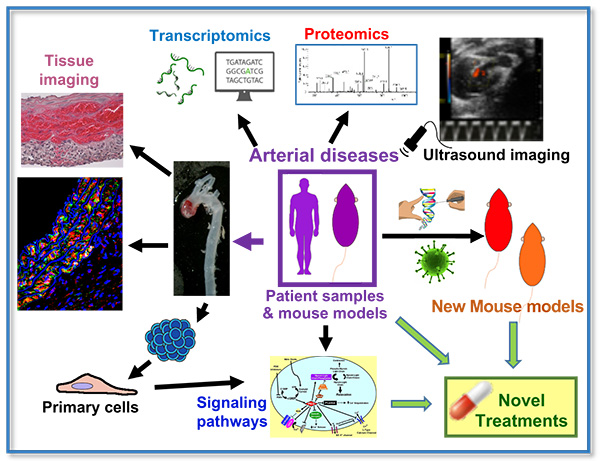
Figure 1. Transcriptomics, proteomics, tissue staining analysis, and primary cell cultures of mouse models of disease and/or patient-derived samples are used to dissect the major signaling pathways that cause arterial diseases, such as aortic aneurysm and arterial hypertension, and identify novel targets for therapeutic intervention. Genetic manipulation and in vivo lentiviral-mediated transduction are then used to generate new mouse models for validation of these targets. The efficacy of novel treatments is assessed longitudinally in vivo by ultrasound imaging of the heart and vessels and ex vivo by tissue analysis.
MOLECULAR BASIS OF LYMPHOMAGENESIS
Immortalization is essential but insufficient for tumor transformation. Lymphoblastoid B-Cell Lines (LCLs), resulting from the infection of normal B lymphocytes from healthy donors with the Epstein-Barr virus, are an example of immortalized non-tumoral cells. Contrary to lymphoma or leukemia cells (tumor cells), LCLs and primary lymphocytes from healthy donors do not grow upon their inoculation in immunodeficient mice and do not grow in soft gels. Just a minor fraction of cancer cells within a tumor has the capacity to initiate a tumor, the Tumor Initiating Cells (TICs). TICs, which can be selected from a pool of cancer cells by culturing them in three-dimensional (3D) soft gels, are particularly resistant to current chemotherapy or radiotherapy, highlighting the utility of soft hydrogels for TICs research. We are interested in identifying genes critical for lymphoid tumor growth in soft gels because they might be also critical for TICs growth in vivo and hence facilitate the design of therapies that efficiently target tumor initiating cells. By comparing lymphoma and LCLs gene expression profiles we have identified > 1600 differentially expressed genes. We found that one of these genes, CDCA7, is overexpressed in lymphoid tumor cell lines and lymphoma biopsy specimens from patients relative to LCLs and control tissues, respectively. We showed that its elevated expression is required for lymphoma/leukemia growth in soft gels, but not in liquid media, for tumor formation in immunodeficient mice, and for lymphoma migration and invasion through its capacity to modulate tubulin and actomyosin cytoskeleton dynamics. With funds from the Asociación Española Contra el Cáncer Foundation we are investigating the mechanisms underlying the differential growth capacity of lymphoma and LCLs in soft gels and have recently started an unbiased high-throughput screening of the capacity of all human proteins to mediate the growth of lymphoid tumor cells in soft gels. We will determine the role of selected proteins in the formation of several lymphoid neoplasia types. With due caution our results could identify proteins involved in TICs proliferation, and prompt the use of drugs or small molecules to modulate them as new therapeutic alternatives for lymphoid tumors.
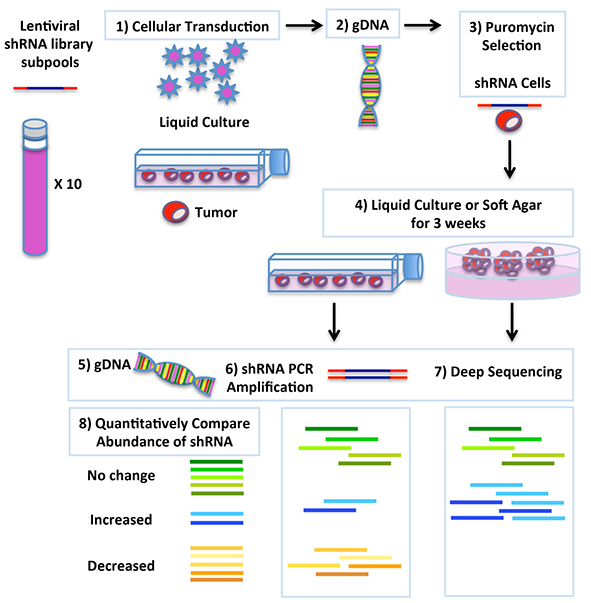
Figure 2. Experimental design scheme of pooled shRNA library screen strategy coupled with deep-sequencing to uncover genes involved in tumor initiating cells growth.

| Last name | Name | Laboratory | Ext.* | Professional category | |
|---|---|---|---|---|---|
| Campanero García | Miguel R. | 227 | 4554 | mcampanero(at)cbm.csic.es | E. Investigadores Científicos de Organismos Públicos |
| Clemente Toribio | Cristina | 227 | 4587 | cristina.clemente(at)cbm.csic.es | Titulado Superior Grado de Doctor |
| Gil Ruiz | Teresa | 227 | 4587 | Becario JAE Intro | |
| Gutiérrez Martínez | Carolina | 227 | 4587 | carolina.gutierrez(at)cbm.csic.es | Titulado Sup. Actividades Tecn. y Prof.GP1 |
| Hernández Alcántara | Alberto | 227 | 4587 | ahernandez(at)cbm.csic.es | M2 |
| Redondo Moya | Juan Miguel | 227 | 4401 | jmredondo(at)cbm.csic.es | E. Profesores de Investigación de Organismos Públicos de Investigación |
| Reyes Campos | Oliva | 227 | 4554 | Estudiante | |
| Sun | Yilin | 227 | 4587 | yilin.sun(at)cbm.csic.es | M2 |
Relevant publications:
- de la Fuente-Alonso, M. Toral, A. Alfayate, M.J. Ruiz-Rodríguez, E. Bonzón-Kulichenko, G. Teixido-Tura, M.J. Méndez-Olivares, D. López-Maderuelo, I. González-Valdés, E. García-Izquierdo, S. Mingo, C.E. Martín, L. Muiño-Mosquera, J. De Backer, J.F. Nistal, A. Forteza, A. Evangelista, J. Vázquez, *M.R. Campanero, and *JM Redondo. *Co-senior & corresponding authors. Aortic disease in Marfan syndrome is caused by overactivation of sGC-PRKG signaling by NO. Nat. Commun. (2021) 12(1): 2628.
- Toral, A. de la Fuente-Alonso, *M.R. Campanero, and *J.M. Redondo. *Co-senior & corresponding authors. The Nitric Oxide Signalling Pathway in Aortic Aneurysm and Dissection. Brit. J. Pharmacol. (2021) doi: 10.1111/bph.15694.
- Martín-Cortázar, Y. Chiodo, R. Jiménez-P., M. Bernbé, M.L. Cayuela, T. Iglesias, and M.R. Campanero. CDCA7 finely tunes cytoskeleton dynamics to promote lymphoma migration and invasion. Haematologica. (2020) 105(3): 730-740.
- S Villahoz, PS Yunes-Leites, N Méndez-Barbero, K Urso, E.Bonzon-Kulichenko, S Ortega, J Vazquez, S Offermanns, *JM Redondo, and *MR Campanero. *Co-senior & corresponding authors. Conditional deletion of Rcan1 predisposes to hypertension-mediated aortic intramural hematoma and subsequent aneurysm and lethal dissection. Nat Commun (2018) 9(1): 4795.
- Jiménez-P., C. Martín-Cortázar, O. Kourani, Y. Chiodo, R. Cordoba, M.P. Domínguez-Franjo, J.M. Redondo, T. Iglesias, and M.R. Campanero. CDCA7 is a critical mediator of lymphomagenesis that selectively regulates anchorage-independent growth. Haematologica. (2018) 103(10): 1669-1678.
- Oller, N. Méndez-Barbero, E.J. Ruiz, S. Villahoz, M. Renard, L.I. Canelas, A.M. Briones, R. Alberca, N. Lozano-Vidal, M.A. Hurlé, D. Milewicz, A. Evangelista, M. Salaices, J.F. Nistal, L.J. Jiménez-Borreguero, J. DeBacker, *M.R. Campanero, and *J.M. Redondo. *Co-senior & corresponding authors. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat. Med. (2017) 23(2): 200-212 Destacado por Science: http://science.sciencemag.org/content/355/6324/twil; Destacado por Biocentury: Inducible nitric oxide synthase 2 (NOS2; iNOS).
- Alvaro-Blanco, Y. Chiodo, K. Urso, C. Martín-Cortázar, O. Kourani, M. Rodriguez-Martinez, E. Calonge, J. Alcamí, J.M. Redondo, T. Iglesias, and M.R. Campanero. MAZ induces MYB expression during the exit from quiescence via de E2F site in the MYB promoter. Nucleic Acids Res. (2017) 45(17): 9960-9975.
- Méndez-Barbero, V. Esteban, S. Villahoz, A. Escolano, K. Urso, A. Alfranca, C. Rodríguez, S.A. Sánchez, T. Osawa, V. Andrés, J. Martínez-González, T. Minami, *J.M. Redondo, and *M.R. Campanero *Co-senior & corresponding authors. A major role for RCAN1 in atherosclerosis progression. EMBO Mol. Med. (2013) 5:1901-1917.
- Molina-Privado, R. Jiménez-P., S. Montes-Moreno, Y. Chiodo, M. Rodríguez-Martínez, L. Sánchez-Verde, T. Iglesias, M.A. Piris, and R. Campanero. E2F4 plays a key role in Burkitt lymphoma tumorigenesis. Leukemia. (2012) 26(10): 2277-2285.
- V Esteban, N Méndez-Barbero, LJ Jiménez-Borreguero, M Roqué, L Novensá, AB García-Redondo, M Salaices, L Vila, ML. Arbonés, *R. Campanero, and *JM Redondo. *Co-senior & corresponding authors. Regulator of calcineurin 1 mediates pathological vascular wall remodeling. J. Exp. Med. (2011) 208: 2125-2139. Seleccionado por Faculty of 1000 (Muller W: 2012. F1000.com/13418975)
Doctoral theses:
- Irene Molina Privado (2009). Estudio de las bases moleculares de la formación del Linfoma de Burkitt para la identificación de posibles marcadores diagnósticos y dianas terapéuticas. Universidad Autónoma de Madrid. Director: Miguel R. Campanero
- Lorena Martínez Gac (2009). Estudio de los mecanismos implicados en la regulación transcripcional de los genes CCNG2 Y C-MYC. Universidad Autónoma de Madrid. Directores: Miguel R. Campanero y Ana Clara Carrera.
- Raúl Jiménez Pérez (2013). Identificación y caracterización funcional de genes implicados en la transformación maligna del compartimento linfocitario. Universidad Autónoma de Madrid. Director: Miguel R. Campanero.
- Nerea Méndez Barbero (2014). Estudio del papel de calcineurina y Rcan1 en el remodelado patológico de la pared vascular. Universidad Autónoma de Madrid. Directores: Miguel R. Campanero y Juan Miguel Redondo.
- Yuri Chiodo (2017). Cell death, cell growth and cell cycle regulation by the Retinoblastoma family. Universidad Autónoma de Madrid. Directores: Miguel R. Campanero y Juan Miguel Redondo.
- Jorge Oller Pedrosa (2017). Identification of the metalloproteinase Adamts1 and Nitric Oxide as new therapeutic targets in aortic diseases. Universidad Autónoma de Madrid. Directores: Miguel R. Campanero y Juan Miguel Redondo.
- Silvia Villahoz Lázaro (2018). Effects of conditional deficiency of Rcan1 in pathological vascular wall remodeling (Tesis Europea). Universidad Autónoma de Madrid. Directores: Miguel R. Campanero y Juan Miguel Redondo.
- Carla Martín Cortázar (2019). Invasión, migración y dinámica del citoesqueleto de células de linfoma mediadas por CDCA7. Universidad Autónoma de Madrid. Director: Miguel R. Campanero.
- Omar Kourani Méndez (2019). Papel del sistema del glutatión en la adaptación y resistencia de los tumores linfoides al estrés oxidativo. Universidad Autónoma de Madrid. Director: Miguel R. Campanero.
Licensed patents:
- Procedimiento de identificación de pacientes con linfoma de Burkitt esporádico, procedimiento de identificación y uso de compuestos para el tratamiento de linfoma de Burkitt esporádico. I. Molina-Privado y Miguel R. Campanero. PCT/ES2008/070182. País de prioridad: Internacional salvo USA. Fecha: octubre de 2007. Entidad titular: CSIC/UAM; Licenciada a BIOTOOLS Biotechnological & Medical Laboratories S.A. el 29 de abril de 2008.
- In vitro method for identifying thoracic aortic aneurysms (TAA) in a subject. J.M. Redondo, J. Oller, N. Méndez-Barbero y Miguel R. Campanero. PCT/EP2016/082925. País de prioridad: Internacional excepto USA. Fecha de prioridad: marzo de 2016; Entidad titular: CNIC/CSIC/UAM. Licenciada a SPHERIUM BIOMED S.L. el 21 de septiembre de 2018. Patente USA (Nº 16/083,165) concedida el 16 de septiembre de 2020.




















