Department Lead

Sundary Sormendi Gómez
Contact information
91 196 44 46
transferencia.con@cbm.csic.es
Our multidisciplinary teams carry out research across a wide range of fields, including viral infections, cancer, neurodegeneration, tissue and organ development, agrotechnology, and bioprocesses. Our goal is to provide innovative solutions to the challenges faced by society.
Explore our protected technology portfolio and contact our Knowledge Transfer Department for more information.
First-in-class antiviral compound against HSV-1 and HSV-2 aciclovir resistant strains
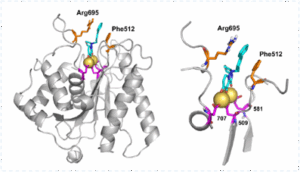 |
LN-7 is a new endonuclease Inhibitor that prevents Herpes Simplex Virus 1 and 2 (HSV-1 and 2) replication in preclinical in vitro and in vivo studies, postulating as novel antiviral candidate for prevention, conventional antiviral resistant HSV and combination therapy against herpesvirus. |
|
Intelectual property Priority patent application filed |
Stage of developement pre-clinical in vivo |
Intended collaboration Licensing and/or co-development |
Contact Sundary Sormendi transferencia.con@cbm.csic.es |
Unmet need
- 64% of population <50yo suffer with HSV-1, totaling 3,8 billion people worldwide.
- Acyclovir remains the most prescribed HSV drug worldwide with over 3,8 million prescriptions in 2023. While 1% of healthy individuals show acyclovir resistance, this increase up to 15% in immunocompromised patients. Second-line treatments, such as cidofovir and foscarnet, are available for drug-resistant cases but have significant side effects.
- Global HSV treatment market was estimated at USD 2.47 billion in 2023, expected to grow to 4.08 billion by 2030 at a CAGR of 8,1%.
Innovative Solution
Ln-7 is a first-in-class antivarial against HSV-1 by targeting the pUL15 endonuclease, a component of the viral packaging motor/terminase complex, using substituted polycyclic pyridones derived from baloxavir acid. Preclinical in vivo and in vitro studies showed:
- LN-7 was equally effective against acyclovir-resistant HSV-1 and HSV-2 strains, showing EC50 values of 3.2 and 3.6 µM respectively.
- Combined administration of LN-7 and acyclovir showed robust synergism with 90% of viral inhibition, and a favorable dose reduction index above 1
- LN-7 exhibited excellent pharmacokinetic and safety profiles for intravenous and oral administration in in vivo (mice and rat).
- Prophylactic administration of LN-7 showed 2-fold lower HSV-1 titers in infected mice.
Technology Highlights
- Novel mode of action, different from other anitvirals.
- Prophylactic antiviral effect.
- Suitable for acyclovir resistant HSV-1 and HSV-2.
- Synergestic acitivity in combination with acyclovir.
- Strong candidate for immunocompromised patiens.
- Safety and efficacy proven in pre-clinical in vivo models.
|
|
New peptides to treat and prevent cognitive deficiency related to age-related disorders
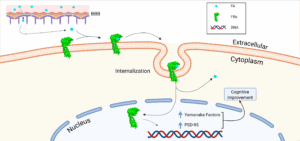 |
Our novel Folate Receptor alpha (FRα) binding peptides induce brain cell reprograming and rejuvenation, enhancing cognitive function in aged mice. The unique properties of our peptides ensure specific binding to FRα preventing from any side effects, and are able to cross blood-brain barrier enabling versatile administration routes. |
|
Intelectual property Priority patent application filed |
Stage of developement pre-clinical in vivo |
Intended collaboration Licensing and/or co-development |
Contact Sundary Sormendi transferencia.con@cbm.csic.es |
Unmet need
- Age-related diseases affecting neuronal functions are on the rise due to an aging population, affecting > 60million people above 65yo, and costing USD1.3 trillion annually worldwide.
- Decreased folate levels is related to aging, with around 30% prevalence of folate deficiency in elderly. Indeed, folate deficiency is link to higher risk of dementia and increased mortality.
- Folate supplement is prescribed as treatment to reduce memory impairment in the elderly. However, its use is limited due to toxic side effects associated with high uptake.
- The global folate supplement market was estimated at USD 11.49 billion in 2024, expected to grow to USD 16.86 billion by 2032, at a CAGR of ~4.9%.
Innovative Solution
Our patented approach includes six new FRα-binding peptides that specifically bind to FRα inducing its translocation into the nucleus and activation as transcription factor. In vitro and in vivo studies showed:
- Expression of Yamanaka factors Sox2 and Klf4 implicated in pluripotency and cell proliferation.
- Increased protein levels of PSD95 implicated in synaptic transmission and cognition through GluN2B (subunit of NMDA receptor) in cortical neurons.
- Reduced density of perineuronal nets (PNN) in the hippocampus and somatosensory neocortex.
- Notable improvement in new object recognition memory, particularly in the long term but also in short-term spatial memory.
- Ability to cross blood-brain barrier allowing versatile administration routes, including intracranial and peritoneal injection, and intragastric inoculation.
Technology Highlights
- Known mode of action, no side effects in pre-clinical mouse models.
- FRα specificity, preventing from toxix high-doce folate effect.
- BBB permeability, enabling versatile administration routes.
- Safety and efficacy proven in pre-clinical mouse models.
|
|
Biotin & Thiamine: A novel therapeutic strategy for Huntington’s Disease
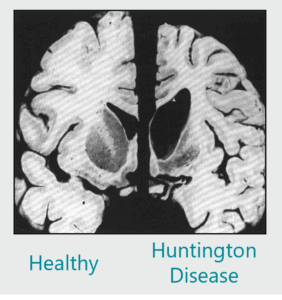 |
Thiamine metabolism is impaired in Huntington Disease (HD). Our in-vivo studies showed that treatment with thiamine and biotin rescues HD symptoms. We are currently evaluating safety and tolerability of thiamine – biotin treatment for HD in a multicentric clinical trial. |
|
Intelectual property Patent granted EP, US |
Stage of developement clinical phase I/II |
Intended collaboration Licensing and/or co-development |
Contact Sundary Sormendi transferencia.con@cbm.csic.es |
Unmet need
- Progressive, and fatal neurodegenerative disorder caused by a genetic mutation leading to toxic function of Huntingtin protein.
- With a prevalence of 1 in 10,000 individuals is one of the most common rare diseases. There is no available cure. Current marketed solutions are just symptomatic and are associated with numerous side effects. There is no EMA approved therapy available.
- Global HD treatment market size was estimated at USD 500 million in 2024 and is projected to reach USD 1,871.2 million by 2030 (23.8% CAGR).
Innovative Solution
- Studies using mouse models and samples from patients showed impaired thiamine metabolism in HD, due to decreased expression of brain specific thiamine transporter ThTr2 (SLC19A3).
- High doses of thiamine and biotin attenuate HD pathology by:
- Restoring brain bioenergetics (TPP levels) and increasing SLC19A3 transporter expression.
- Preventing striatal atrophy, attenuating neuropathology, and improving motor coordination.
- Pre-clinical data eligible for Orphan Drug Designation (ODD).
- On going phase I/II multicentric clinical trial to evaluate safety and tolerability of thiamine and biotin treatment for HD (NCT04478734).
Technology Highlights
- Improvement of HD neuropathology, radiology and motor symptoms in mouse model
- Proven safety and good tolerability in human
- Good treatment adherance in human
- ODD eligible
- Allows combination therapy with other treatments
|
|
Biomarker for diagnosis and prognosis of Multiple Sclerosis (MS)
 |
TAF1 isoform, where C-terminal end is loss, dysregulates expression of genes implicated in Multiple Sclerosis (MS) neuropathology. The detection of this isoform in blood and/or cerebrospinal fluid may be used as a biomarker for diagnosis and prognosis of MS. The novel mouse line expressing this isoform can be used to validate new MS therapies. |
|
Intelectual property Priority patent application filed |
Stage of developement pre-clinical in vivo |
Intended collaboration Licensing and/or co-development |
Contact Sundary Sormendi transferencia.con@cbm.csic.es |
Unmet need
- Multiple sclerosis is a chronic autoimmune disease of unknown etiology characterized by neuroinflammation, demyelination and axonal damage, affecting 2.9 million people worldwide.
- 40% of patients are diagnosed >5 years from first MS symptoms.
- Although modifying disease treatments are available in the first stages, there is no cure.
- Main challenges for efficient diagnosis and treatment are related to a lack of:
- understanding of the complex molecular basis of the disease
- early diagnosis and prognosis of MS progression biomarkers
- pre-clinical models for new therapies R&D
Innovative Solution
Our preclinical studies identify a TAF-1 isoform missing the C-terminal end (TAF1ex38) as a candidate biomarker for diagnosis and prognosis of multiple sclerosis (MS).
- Normal-appearing grey and white matter (NAG+WM) from MS patients showed reduced presence of TAF1ex38
- Reduced levels of TAF1ex38 can be explained by increased cathepsin B activity outside the lysosomes in brain tissue from MS patients
- Decreased TAF1ex38 levels were related to MS progression
- First mouse model of progressive MS, where deletion of TAF1ex38 mimics MS pathophysiology.
- The C-terminal end of TAF1 interplays with multiple transcriptional regulators involved in RNAPII transcriptional initiation and pause release, and its absence leads to downregulation of oligodendroglia genes, and upregulation of inflammatory genes.
Technology Highlights
- Novel biomarker for diagnosis and prognosis
- Minimally invasive method through blood or CFS extraction
- Known molecular mechanism of action
- Novel pre-clinical model for MS
|
|
|
Targeted Immunotherapy for T-Cell Acute Lymphoblastic Leukemia (T-ALL)
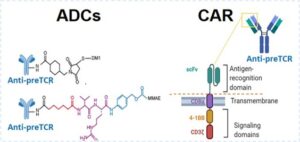 |
A new immunotherapy strategy based on the administration of ADCs or CAR-T cells derived from a monoclonal antibody targeting the pre-TCR receptor, which impairs LIC activity and tumor progression, has been developed and validated in a preclinical human T-ALL xenotransplantation model in mice. |
|
Intelectual property PCT application filed |
Stage of developement pre-clinical in vivo |
Intended collaboration Licensing and/or co-development |
Contact Sundary Sormendi transferencia.con@cbm.csic.es |
Unmet need
- T-cell acute lymphoblastic leukemia (T-ALL) has aggressive progression and poor prognosis in relapsed patients.
- 65% early relapse upon front-line therapy (before 18 motnhs) with <10% long-term survival.
- Current treatments rely on chemotherapy and stem cell transplantation, which offer limited specificity and high toxicity.
- Targeted immunotherapies like CAR-T and monoclonal antibodies face challenges due to lack of tumor-specific targets, leading to T-cell aplasia and CAR-T fratricide.
- The global T-ALL treatment market was estimated at USD 3.3 billion in 2023, expected to grow to USD 5.4 billion by 2035, at a CAGR of ~4.19%.
Innovative Solution
Our patented technology targets pre-TCR, a receptor expressed in >50% of T-ALL cases but absent in healthy peripheral T cells, enabling selective targeting of malignant cells, including those displaying Leukemia Initiating Cell (LIC) activity.
- Dual therapeutic approach:
- Antibody-Drug Conjugates (ADCs) for targeted toxin delivery
- CAR-T cells engineered to recognize pre-TCR
- Comparative Advantage:
-
- Unlike CD7/CD5 CAR-T therapies, which carry the risk of life-threatening T-cell aplasia due to shared antigen expression, pre-TCR targeting avoids healthy T-cell depletion.
- Demonstrated superior selectivity and efficacy in preclinical xenograft models.
- Dynamic internalization of pre-TCR enhances ADC uptake and therapeutic potency.
Technology Highlights
- Selective targeting of leukemia-initiating cells (LICs), key to impair relapse
- Avoids life-threatening T-cell aplasia and CAR-T fratricide
- Low risk of escape mutants due to target’s role in T-ALL cell survival
- Validated in human xenograft mouse models
|
|
