Scientific facilities
Flow cytometry
Facility Head

Berta Raposo
Scientific Lead

María Luisa Toribio
Contact details
91 196 44 99
citometria (at) cbm.csic.es
Booking system
Reagents
Fees
At the Flow cytometry facility of the CBM, our staff provides scientific-technical support on flow cytometry, develops and optimizes new applications and performs cell separations.
Flow cytometry is an analytical tool that allows discrimination of particles of different size and colour. It is an analytical and/or preparative technique based on the simultaneous analysis of several parameters of optical nature, quickly and on a large number of individualized particles. This analysis is performed on each particle based on light scattering and fluorescence emission of particles illuminated by a light source.
The parameters that can be analyzed by flow cytometry are: i) those related to the physical characteristics of the particle: size and complexity and ii) the different fluorescence associated with the particle.
The analysis is performed at rates of thousands of cells/second, which allows obtaining data of high statistical reliability, as well as identifying populations represented at low frequency within the overall population.
Another important feature of flow cytometry is that the analysis is performed in a particle suspension, so the particles or cells under analysis must be in suspension and in the form of a single particle (cell).
Finally, it is important to note that there is a restriction on the size of the particles under analysis, which must be between 0.5 and 100 μm.
Vacío
Conventional cytometry
|
FACSCalibur (Becton Dickinson)
Software: Cell Quest-Pro |
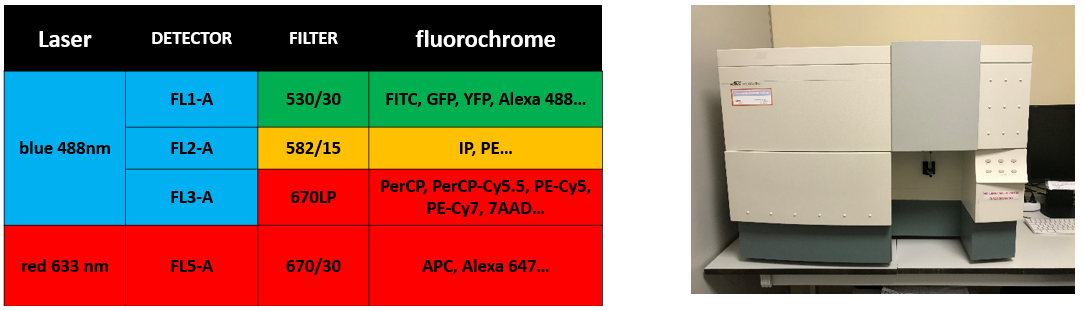 |
|
FACSCanto A (Becton Dickinson)
Software: DiVA 8.0.1 |
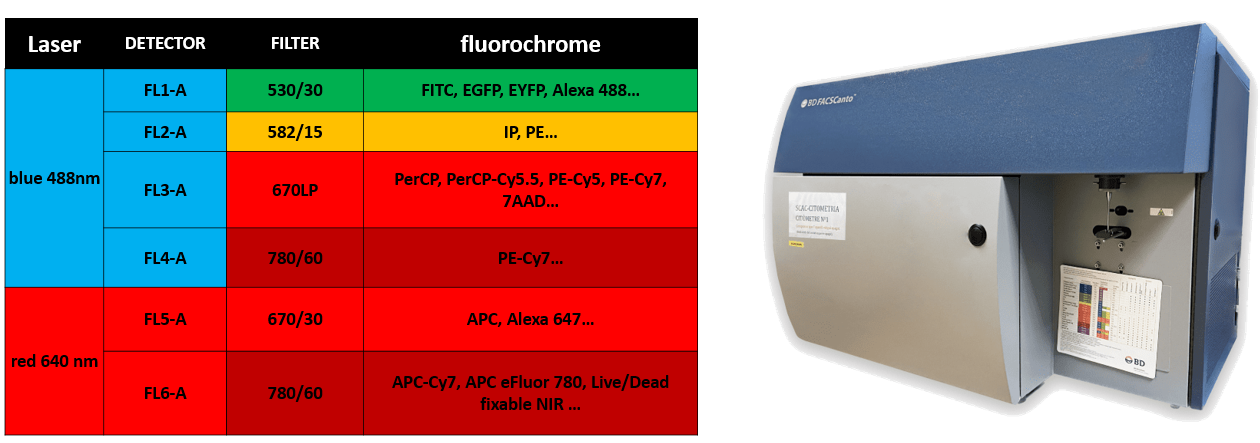 |
|
FACSCanto II High Throughput Sampler Option (Becton Dickinson)
4 detectors for the 488nm laser (blue): 530/30, 585/42, 695/40, 780/60 Software: DiVA 8.0.1 |
 |
|
FACSCanto II High (tubes only) (Becton Dickinson) Software: DiVA 8.0.1 |
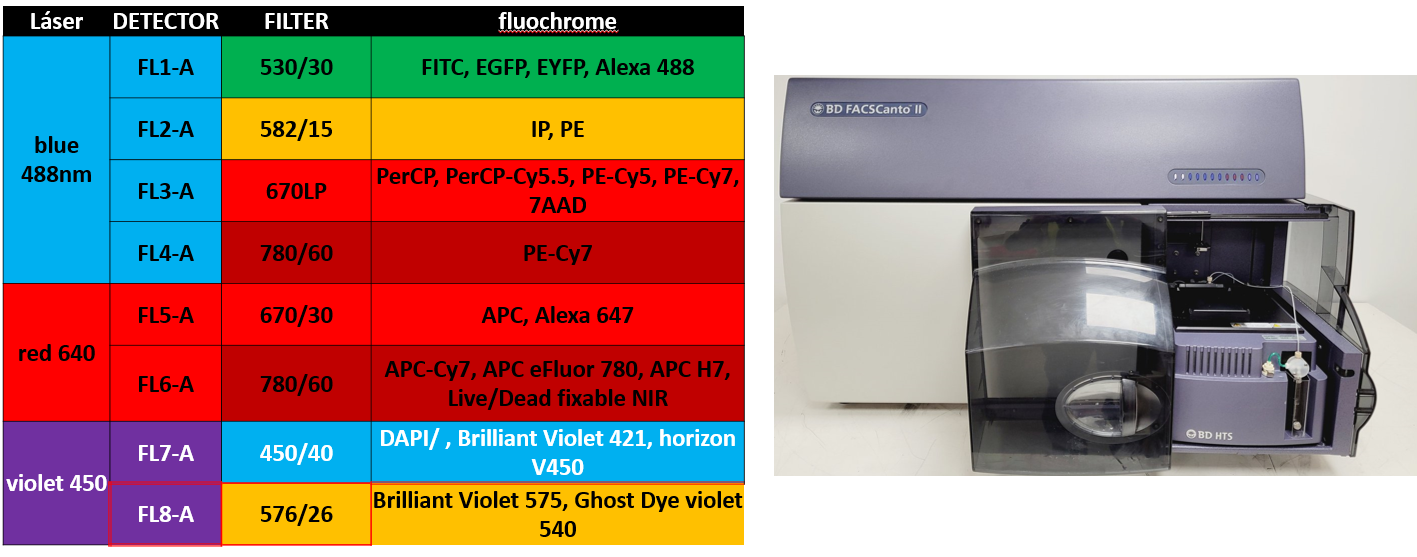 |
Spectral cytometry
| AURORA 4L (Cytek) Optical Configuration: 4L (16V-14B-10YG-8R) 4 laser lines and 48 detectors: 16 detectors for the violet laser (405nm), 14 detectors for the blue (488nm), 10 detectors for the yellow green (561nm) and 8 detectors for the red (633nm). Technical Characteristics: This equipment uses full spectrum technology to provide unprecedented flexibility to users, enabling them to use of a wide range of new fluorochrome combinations without reconfiguring the system for each application. This technology provides high-resolution data at the single-cell level to resolve the most challenging cell populations, such as those with high autofluorescence or low levels of marker expression. |
| AURORA 5L (Cytek) Optical Configuration: 5L (16UV-16V-14B-10YG-8R) 4 laser lines and 64 detectors: 16 detectors for the ultraviolet laser (355nm),16 detectors for the violet laser (405nm), 14 detectors for the blue (488nm), 10 detectors for the yellow green (561nm) and 8 detectors for the red (633nm). Technical Characteristics: This equipment uses full spectrum technology to provide unprecedented flexibility to users, enabling them to use of a wide range of new fluorochrome combinations without reconfiguring the system for each application. This technology provides high-resolution data at the single-cell level to resolve the most challenging cell populations, such as those with high autofluorescence or low levels of marker expression. |
Cell sorters
| FACSAria Fusion (Becton Dickinson) The BD FACSAria Fusion is an easy-to-use cell separator that allows the separation of a large number of samples of different origins. It is installed in a Class II Type A2 biosafety cabinet equipped with an integrated aerosol containment system that operates independently. Some technical features are detailed below: Equipped with four excitation lasers 405 ,488, 561and 640 nm. Eighteen detectors (16 fluorescence + 2 scattering) of which 5 are for the 405nm laser (450/40, 525/50, 610/20, 660/20, 710/50, 780/60), 2 for the 488nm laser: 530/30, 695/20, 4 for the 561nm laser: 610/20, 670/14, 710/50, 780/60 and 3 for the 640nm laser: 670/30, 730/45, 780/60. It is also equipped with a temperature control system and sample agitation. |
 |
Work stations and software
The service provides users with three analysis stations (Hewlett-Packard) equipped with the following software packages:
– Station 1: FlowJo v10 and 7.6.5, Spectroflo3.3.1 (4 or 5 L configuration)
– Station 2: FlowJo v10, Spectroflo 3.3. 1 (4 L configuration)
– Station 3: FlowJo v10, Spectroflo 3.3.1 (5 L configuration), Gen5 and DIVA 9, as well as an online license of OMIQ software (a modern, cloud-based flow cytometry analysis platform bridging machine learning and analytical pipelines with classical analysis).
Other equipment
| Cytation (Agilent) Technical Features: The BioTek Cytation 1 multimode cell imager combines fluorescence and high contrast brightfield imaging with conventional multimode detection. This microscope is equipped with a 4X objective, and 3 fluorescence lines: – DAPI (EX 377/50 nm, EM 447/60 nm); LED 365 nm. – GFP (EX 469/35 nm, EM 525/39 nm); LED 465 nm – RFP (EX 531/40 nm, EM 593/40 nm); LED 523 nm Gen5 software simplifies image capture and plate reading. |
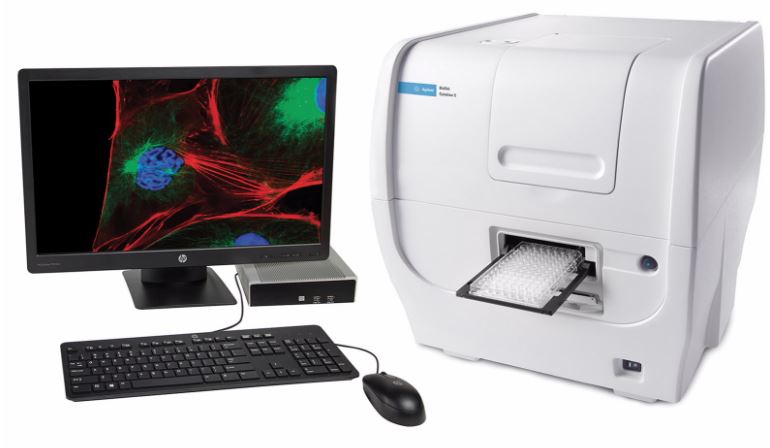 |
| eFlux Analyzer XF96 (Agilent) Technical Features: The Extracellular Flux Analyzer XF96 (Agilent-Seahorse) allows the characterization of the energy metabolism of cultured cells in a non-invasive way. The instrument analyzes oxygen consumption (OCR) and extracellular acidification (ECAR, an indirect measure of lactate production) in 96-well plates. Our service provides access to the equipment as well as basic assistance in both experiment design and data analysis. |
 |
|
NanoSight NS300 (Malvern Panalytical) Applications: |
 |
Staff

Berta Raposo Ponce
Lab.: 111 Ext.: 4499
braposo(at)cbm.csic.es

Raquel Nieto Pintado
Lab.: 111 Ext.: 4698/4705
rnieto(at)cbm.csic.es

Silvia Andrade Calvo
Lab.: 111 Ext.: 4698/4705
sandrade(at)cbm.csic.es

Matilde Riquelme Sánchez
Lab.: 111 Ext.: 4698/4705
mriquelme(at)cbm.csic.es

