Programa Científico
Procesos fisiológicos y patológicos
UNIDADES EN ESTE PROGRAMA
Neuropatología molecular Redes metabólicas y de señalización en la enfermedad
GRUPO DE INVESTIGACIÓN
Metabolismo Energético Traslacional

Laura Formentini
Investigamos el metabolismo energético como posible diana terapéutica en patología. Utilizando modelos de disfunción mitocondrial (ratones con defectos en la fosforilación oxidativa, LowOXPHOS; ratones con disfunción de la oxidación de ácidos grasos, LowFAO), estudiamos cómo factores ambientales (edad, sexo, dietas) afectan al metabolismo a nivel celular, tisular y del organismo, identificando nuevos aspectos mitocondriales que limitan la homeostasis celular.
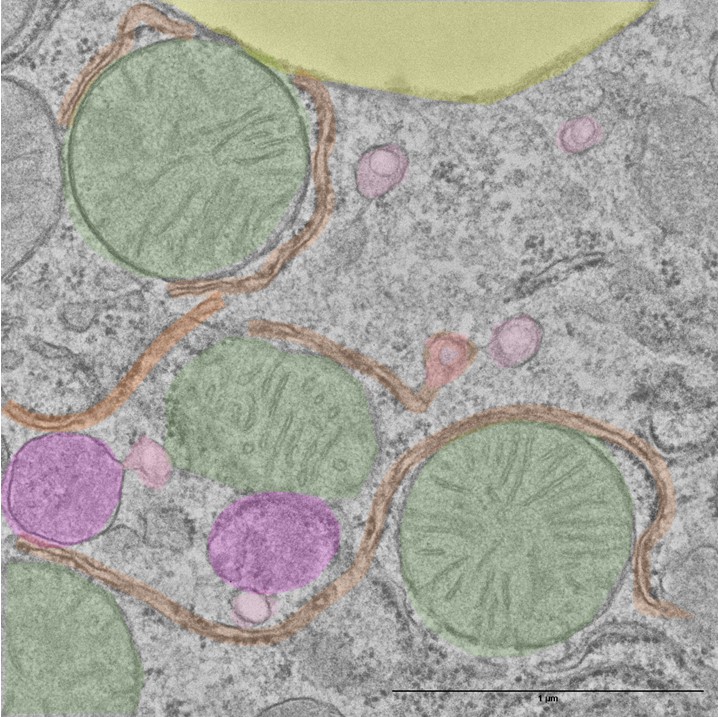
Investigación
Past achievements
During the past years, our research has been focused on understanding how mitochondrial energy metabolism contributes to the integration of cellular functions, leading to the onset and progression of various pathologies. Complex regulatory mechanisms enable mitochondrial metabolism to meet cellular demands, which go beyond ATP production. We have demonstrated that mitochondrial oxidative phosphorylation also plays additional roles in controlling cell immunity and inflammation (Formentini L. et al., Cell Reports, 2017, PMID: 28494869) and regulating intra- and inter-cellular oncogenic signals (Nuevo-Tapioles, C. et al., Nature Communications, 2020, PMID: 32681016). Impaired mitochondrial function also significantly affects adipose tissue and skeletal muscle lipid species and metabolism (Formentini L et al., Diabetologia, 2017, PMID: 28770317; Sanchez-Gonzalez C et al, EMBO J. 2020, PMID: 32488939). Interestingly, these metabolic disturbances impair ROS and calcium signaling, leading to profound changes in muscle structure (Sanchez-Gonzalez C et al, Cell Death and Disease 2022, PMID: 32488939), thus emerging as key hallmarks of myopathies. Very recently, we have demonstrated the existence of a metabolon in skeletal muscle, aimed at integrating nutrient catabolism with mitochondrial efficiency (Nat Metabolism 2024, doi: 10.1038/s42255-023-00956-y).
Current Aims
One of the main goals of my research line, supported by PID2022-136738OB-I00 national funding and Fundación Ramón Areces, is to further investigate mitochondrial metabolism in pathophysiology. Using two conditional and tissue-specific mouse models with impaired mitochondrial activity (dysfunctional oxidative phosphorylation mice, LowOXPHOS mice; dysfunctional fatty acid oxidation mice, LowFAO mice), our research group is elucidating how different mitochondrial dysfunctions, environmental factors, and diets impact metabolism at the cellular, tissue, and organismal levels. We aim to identify the aspects of mitochondrial activity that limit cell homeostasis and understand which products of metabolism are essential for proper organism function, as well as how cells obtain or transform them in physiological tissue environments. This knowledge is crucial for exploiting mitochondrial metabolism for therapeutic purposes in the field of cancer and ageing.
Miembros del grupo

Laura Formentini
Lab.: 326 Ext.: 4618
lformentini(at)cbm.csic.es

Beñat Salegi Ansa
Lab.: 326 Ext.: 4648
bsalegi(at)cbm.csic.es

Tzoline Topdjian Panosian
Lab.: 326 Ext.: 4648
ttopdjian(at)cbm.csic.es

Jose María Lanza Arnaiz
Lab.: 326 Ext.: 4648
jmlanza(at)cbm.csic.es

Pablo Ignacio Sánchez Aguilera
Lab.: 326 Ext.: 4618
pablo.sanchez(at)cbm.csic.es

Rocío Callado Gil
Lab.: 326 Ext.: 4618

Georgios Niforos Gkarsia
Lab.: 326 Ext.: 4648
georgios.niforos(at)cbm.csic.es

Florian Seeger
Lab.: 326 Ext.: 4648
florian.seeger(at)cbm.csic.es
Publicaciones representativas
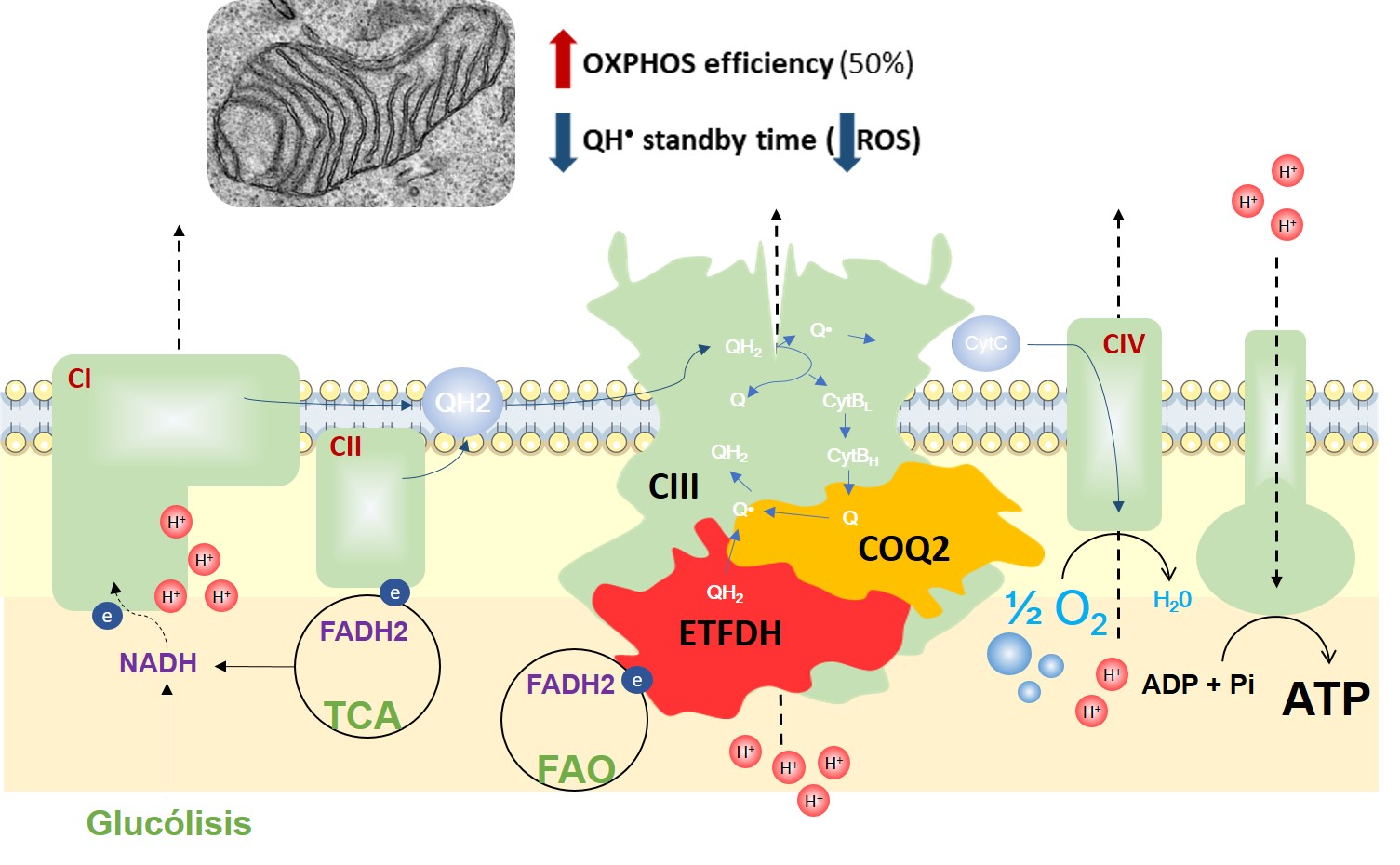
An ETFDH-driven metabolon supports OXPHOS efficiency in skeletal muscle by regulating coenzyme Q homeostasis
Juan Cruz Herrero Martín et al.
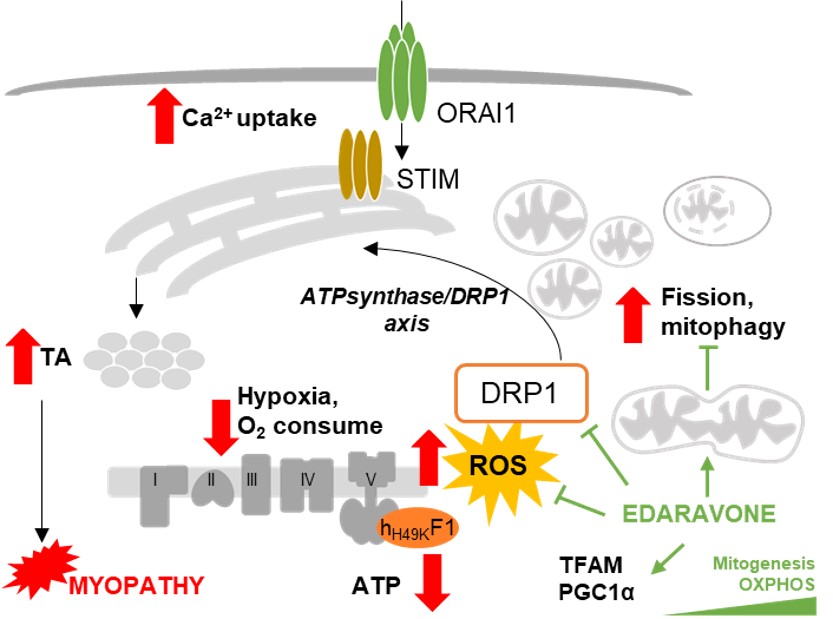
Chronic inhibition of the mitochondrial ATP synthase in skeletal muscle triggers sarcoplasmic reticulum distress and tubular aggregates
Cristina Sánchez-González et al.
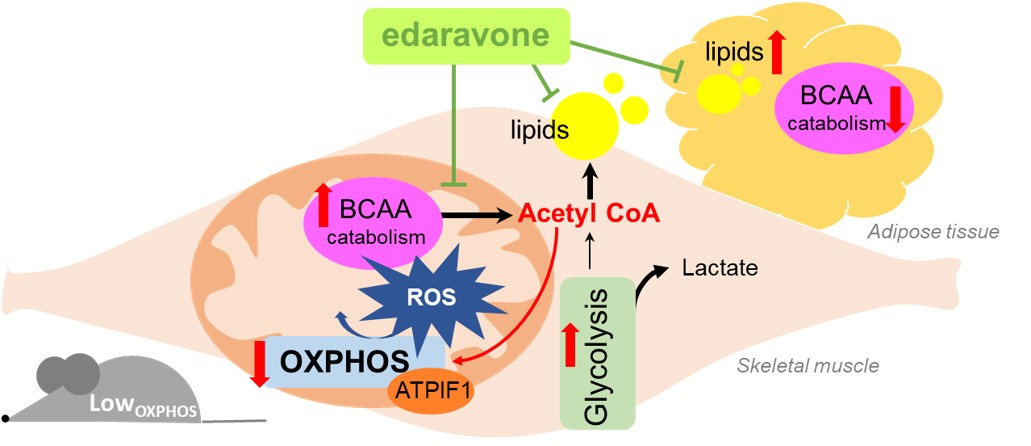
Dysfunctional oxidative phosphorylation shunts branched-chain amino acid catabolism onto lipogenesis in skeletal muscle
Cristina Sánchez-González et al.

Coordinate β-adrenergic inhibition of mitochondrial activity and angiogenesis arrest tumor growth
Cristina Nuevo-Tapioles et al.





