Scientific Program
Genome dynamics and function
RESEARCH GROUP
Telomeres in cancer and regeneration

Ignacio Flores
Telomere shortening during cell division can cause telomere fusions and genomic instability in tumors. Our group investigates the formation and function of these telomere fusions using biochemical, molecular, CRISPR/Cas and genomic analysis techniques. The ultimate goal of our research is the discovery of new markers and treatments against cancer
Research
Telomeres in cancer and regeneration.
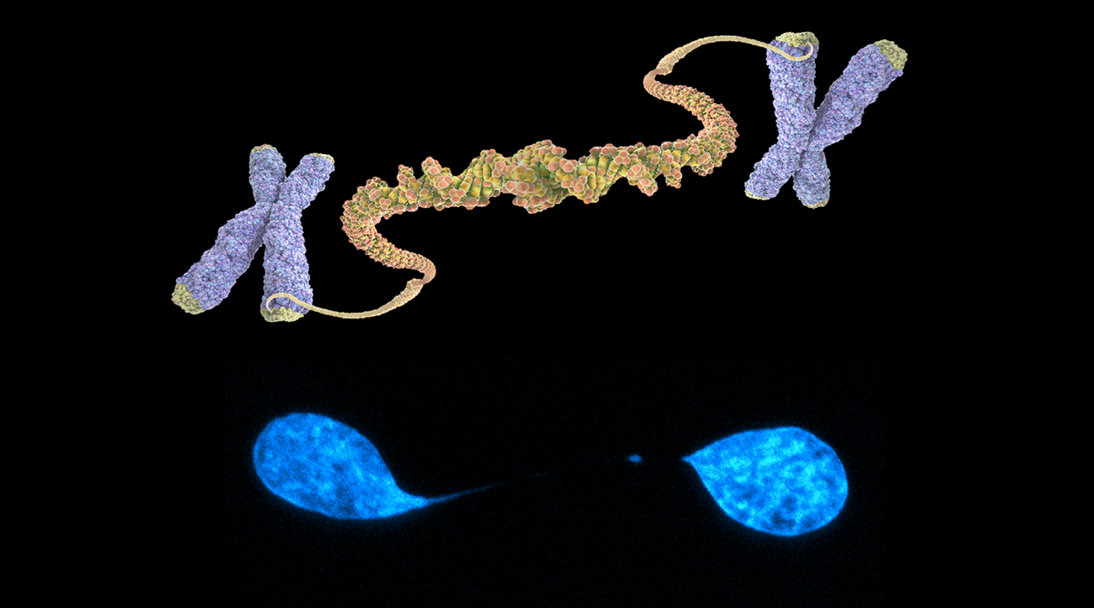
Telomeres are essential components of our genome, protecting it from deterioration and instability. They consist of repetitive DNA sequences and specialized proteins that are located at the ends of our chromosomes. An important aspect of telomere biology is the potential for telomere fusions, which occur when two chromosomes become physically joined at their telomeres. This can trigger a series of genomic rearrangements and oncogenic alterations, ultimately leading to the development of malignant tumors and resistance to chemotherapy. Despite their relevance in tumor evolution, our understanding of the patterns and consequences of telomere fusions in human cancer is still limited. Our group is currently focused on understanding the role of telomeric fusions in cancer and regeneration.
Telomere fusions in cancer. In recent years, we have used whole-genome sequencing data to study the rates and spectrum of somatic telomere fusions (TFs) in over 30 different types of cancer. Our findings indicate that TFs are widespread in human tumors, with rates that vary greatly between and within different types of cancer. In addition to end-to-end fusions, we have identified unique patterns of TFs that are linked to the activity of the alternative lengthening of telomeres (ALT) pathway. One particularly exciting discovery has been the detection of TFs in the blood of cancer patients, offering a potential method for early cancer detection with high specificity and sensitivity. This is especially promising for cancer types such as pancreatic cancer and brain tumors, which currently have limited options for early detection. Through biochemical, molecular, CRISPR/Cas, and genomic (NGS) techniques, we will continue to investigate the occurrence and function of telomere fusions in various human and mouse tumors. Understanding the role of TFs in cancer will be crucial in developing targeted therapies and improving patient outcomes.
Telomere fusions (TF) in cardiomyocyte maturation and heart regeneration. Maturation in many mammalian cells, including cardiomyocytes, is accompanied by an increase in the number of chromosomes, or polyploidization. This increase in chromosome sets may contribute to the limited ability of mature cardiomyocytes to divide after a damage, as polyploid cells are often unable to undergo successful cell division. While the importance of cardiomyocyte polyploidy is well-recognized, the mechanism behind it is not understood. In recent years using a high-content imaging approach, we have discovered that approximately half of postnatal mouse cardiomyocytes that enter mitosis have discernible chromosome bridges. Furthermore, we observed that the ploidy of cardiomyocytes is tracked by an increasing number of fusions between chromosome ends, providing evidence of a direct link between TFs and cardiomyocyte polyploidization. We also found that TFs are a conserved phenomenon, present in the hearts of mice, zebrafish, pigs, and humans. Importantly, zebrafish hearts, which are able to regenerate and have mostly diploid cardiomyocytes, have few TFs. In fact, deleting telomerase to inhibit zebrafish regeneration increased the number of polyploid cardiomyocytes, supporting the idea that cardiomyocyte polyploidization impairs heart regeneration. Together these results identify TFs as a mechanism leading to chromosome bridging and polyploidization and suggest that failed cardiomyocyte cytokinesis originates from a mitotic defect. We are currently investigating whether fine-tuned regulation of telomerase avoids the presence of TFs, increases the number of diploid cardiomyocytes, and extend the cardiomyocyte proliferation and heart regeneration window. Our findings suggest that regulating the presence of TFs may be key to enhancing cardiomyocyte proliferation and heart regeneration.
Group members

Ignacio Flores Hernández
Lab.: 114.2 / 403 Ext.: 4581
iflores(at)cbm.csic.es

Ana María Andreea Badarau Radu
Lab.: 403 Ext.: 4608
andreea.b(at)cbm.csic.es

Irene Álvarez Mejías
Lab.: 403 Ext.: 4608
ialvarez(at)cbm.csic.es

María José Morillo Chincoa
Lab.: 403 Ext.: 4608
maria.morillo(at)cbm.csic.es
Selected publications
The ALT pathway generates telomere fusions that can be detected in the blood of cancer patients
Francesc Muyas et al.
Telomeres Fuse During Cardiomyocyte Maturation
Esther Aix, PhD et al.
Telomeres and telomerase in heart regeneration
Esther Aix et al.
Postnatal telomere dysfunction induces cardiomyocyte cell-cycle arrest through p21 activation
Esther Aix et al.