Scientific Program
Interactions with the environment
RESEARCH GROUP
Development of the human lymphohematopoietic system

Maria Luisa Toribio
Our interest is to decipher how the dysregulation of physiological mechanisms mediating the development of T lymphocytes in the human thymus leads to the generation of T-cell acute lymphoblastic leukemia (T-ALL). The final aim is identifying unique T-ALL targets for developing novel specific immunotherapies, including CAR-T cells, which remains an unmet clinical need in T-ALL.
Research
Our group studies the cellular and molecular mechanisms that control the commitment and differentiation along the T-cell lineage of hematopoietic progenitors seeding the human thymus. Our goal is to obtain mechanistic information about how dysregulation of particular intrathymic physiological pathways leads to T-cell acute lymphoblastic leukemia (T-ALL), with the final aim of identifying specific molecular targets for therapeutic intervention. Focusing on the NOTCH1 pathway as a major driver of T-ALL, we recently developed a novel in vivo model of de novo generation of human T-ALL, which revealed the contribution of several NOTCH1 targets at distinct stages of T-ALL pathogenesis. In the last two years, this information was used together with proprietary monoclonal antibodies (mAbs) and patented technology to develop state-of-the-art therapeutic strategies targeting T-ALL-specific molecules, including chimeric antigen receptors- (CAR-) armed T cells, therapeutic antibody-drug conjugates (ADCs), and secreting T-cell engaging antibodies (STAbs). We also generated cutting-edge preclinical models for in vivo validation of our T-ALL immunotherapy strategies, as an obligatory step preceding clinical translation. Our results have provided promising preclinical results as proof of concept of the suitability of new targets for implementation of next-generation efficacious and safe strategies overcoming current challenges of T-ALL immunotherapy.
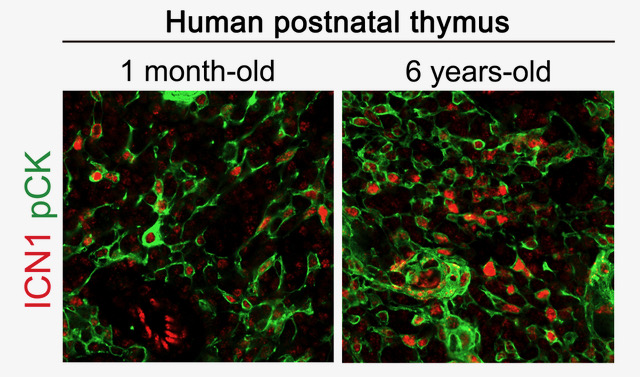
On the other hand, we have studied the spatiotemporal regulation of NOTCH1 activation in the postnatal human thymus and have uncovered a novel role for NOTCH1 signaling in thymus biology, which is not confined to T-cell development. Rather, NOTCH1 signaling is also induced in thymic epithelial cells (TECs), and increases significantly with age, mostly in the medullary TEC (mTEC) compartment, in both humans and mice, suggesting a conserved role for NOTCH1 in TEC homeostasis during thymus aging. Accordingly, we found that organization and integrity of the postnatal thymus critically depends on Notch activation, as specific abrogation of canonical Notch signaling in epithelial cells (Foxn1Cre/+ x RBPjκfl/fl mutant mice), led to a significant disruption of the medullary thymic microenvironment, accompanied by a decrease of mTEC numbers, resulting in an accelerated thymus involution. These data uncover a new role for NOTCH1 activation in the control of adult TEC homeostasis, and point toward Notch signaling manipulation as a novel strategy for thymus regeneration therapies to improve T-cell production in aging and other clinical settings.
Group members

Marina García Peydró
Lab.: 222 Ext.: 4588
mgpeydro(at)cbm.csic.es

María Luisa Toribio García
Lab.: 222 Ext.: 4556
mtoribio(at)cbm.csic.es

Patricia Fuentes Villarejo
Lab.: 222 Ext.: 4588
pfuentes(at)cbm.csic.es

Juan Alcaín Sánchez
Lab.: 222 Ext.: 4588
jalcain(at)cbm.csic.es

Carmela Cela Rodríguez
Lab.: 222 Ext.: 4588
ccela(at)cbm.csic.es

Eloisa Castillo Gutiérrez
Lab.: 222 Ext.: 4588
eloisa.castillo(at)cbm.csic.es

Raquel Victoria Pérez Fernández
Lab.: 222 Ext.: 4588
rvperez(at)cbm.csic.es

Ángela Aparicio Valencia
Lab.: 222 Ext.: 4588
aaparicio(at)cbm.csic.es
Selected publications
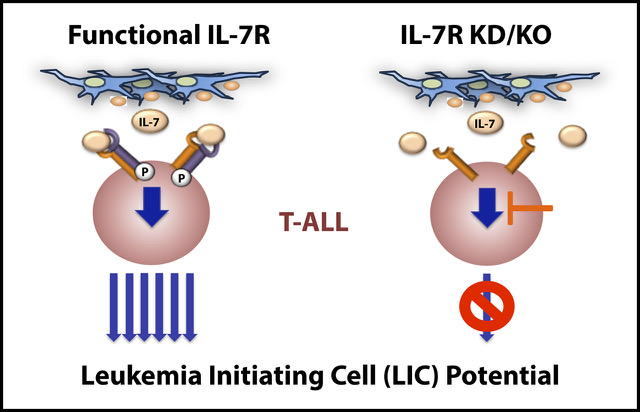
IL-7R is essential for leukemia-initiating cell activity of T-cell acute lymphoblastic leukemia
Sara González-García et al.

The NOTCH1/CD44 axis drives pathogenesis in a T cell acute lymphoblastic leukemia model
Marina García-Peydró et al.

Dynamic regulation of NOTCH1 activation and Notch ligand expression in human thymus development
María J García-León et al.
Spatially restricted JAG1-Notch signaling in human thymus provides suitable DC developmental niches
Enrique Martín-Gayo et al.