Scientific Program
Genome dynamics and function
RESEARCH GROUP
Chromatin, cancer and the ubiquitin system

Emilio Lecona Sagrado
Our research explores the changes in the replisome, the machinery in charge of DNA replication, and their effect in DNA replication dynamics. We study the control of replication factors by ubiquitin and SUMO, and carry out proteomic analysis of replisome composition to determine the role of DNA replication in the control of chromatin structure, cellular identity and cancer.
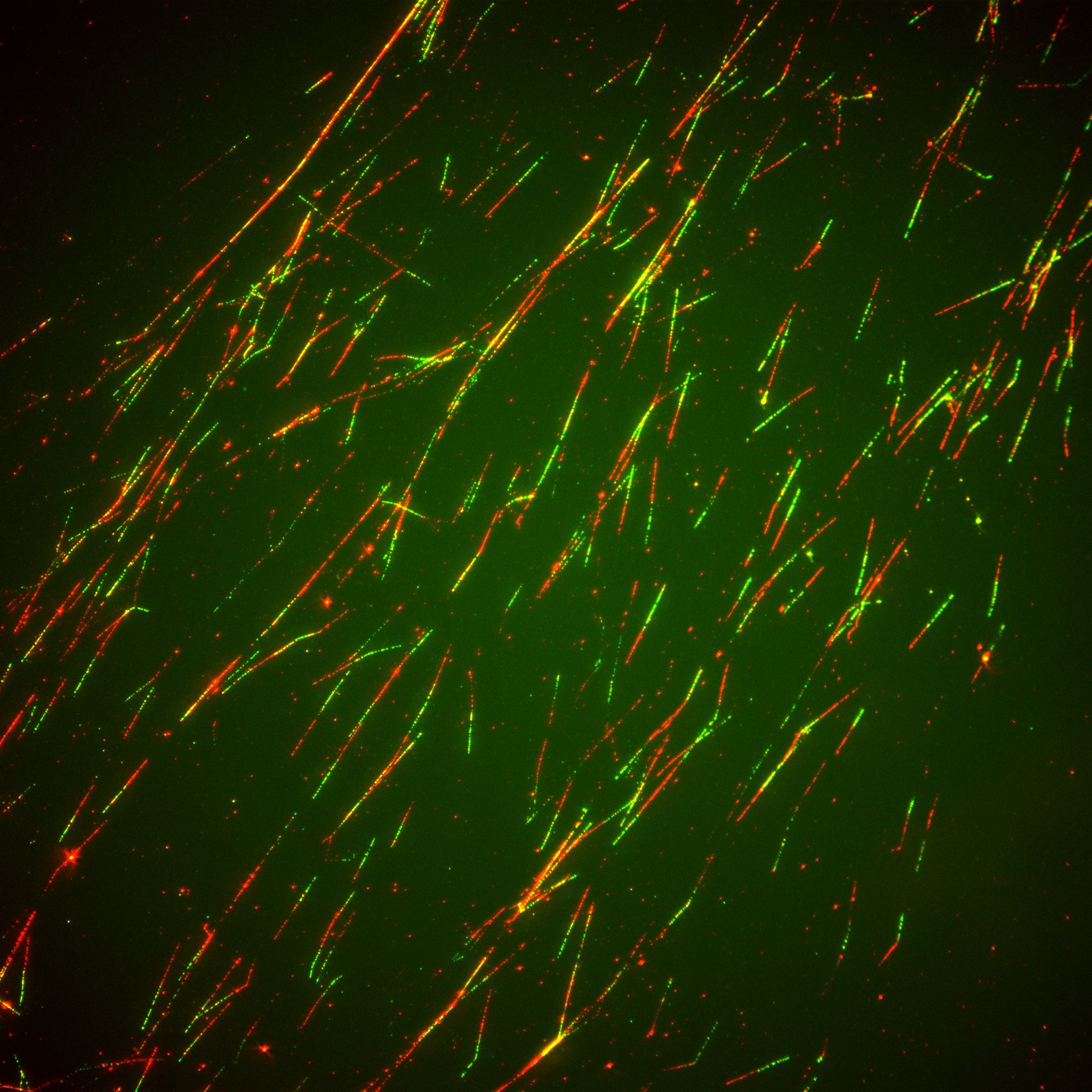
Research
DNA replication is an essential process in cell biology as it mediates the faithful transmission of genetic information through cell division. The copy of the DNA is carried out by a multiprotein complex known as the replisome that coordinates the different activities required to duplicate the genetic material in a cell. Nonetheless, DNA replication is an imperfect process. The mistakes made by the replisome generates mutations during the duplication of the DNA. In addition, the presence of DNA damage generates replication stress and hinders the advance of the replication forks. To prevent the acquisition of mutations and genomic alterations, the replisome incorporates additional factors to repair the DNA and activate the replication stress response. The composition of the replisome also impacts the dynamics of DNA replication, modulating the progression of the forks to deal with the presence of damage or different chromatin states. Thus, the replisome is a malleable and dynamic entity with functions beyond the duplication of the DNA, answering to external input and impacting chromatin itself.
Our group aims at understanding how the composition of the replisome changes and the consequences of these alterations in DNA replication dynamics, chromatin structure and cellular identity, as well as in pathological settings as cancer. On one hand, we explore the replication factors that are subject to dynamic localization to the replisome in S phase, their effect on DNA replication and their regulation. In addition, we are carrying out a proteomic characterization of replisome composition in different settings to establish a collection of replisomes that allows us to understand how specific replication factors impact cellular identity.
We pay special attention to the role of the small protein modifiers ubiquitin and SUMO in the control of replisome components. These modifiers reversibly control the activity, localization and stability of target proteins. Nascent chromatin is a SUMO rich environment and the maintenance of this landscape is essential for DNA replication leading to the proposal that the group SUMOylation of the replisome is required for its functions (Lecona and Fernández-Capetillo, Bioessays 2016). We have shown that the ubiquitin/SUMO landscape during DNA replication requires the joint action of USP7 as SUMO dependent deubiquitinase and the AAA+ ATPase VCP/p97 that extracts ubiquitylated and SUMOylated proteins from chromatin in S phase (Lecona et al., NSMB 2016; Franz et al., Cell Rep. 2021). Following this line of research, we want to understand how DNA replication works in the absence of the SUMO rich environment. In addition to the group modification of the replisome, ubiquitin and SUMO serve as molecular timers that control the activation of presence of these factors in the replisome (Martín-Rufo et al., SCDB 2022). We have identified the DNA polymerase /Primase complex as a new substrate for VCP/p97. The extraction of this complex from chromatin restricts the number of Okazaki fragments and the basal activation of the replication stress response. We are looking for new replication factors targeted by VCP/p97 and we want to understand why their presence in the replisome needs to be regulated.
In the systematic analysis of replisome composition we are focusing on three complementary settings. First, we analyze DNA replication through different chromatin environments. Then, we also compare replisome composition in stem versus differentiated cells, since they display very different DNA replication dynamics. Last, we are interested in understanding the changes in replisome composition that come along and enable the process of malignant transformation. The establishment of a “collection of replisomes” is the first step to understand the role that DNA replication plays in the control of chromatin, the establishment of cell identity and its loss in cancer. We explore DNA replication as an active determinant of chromatin with functions beyond the mere transmission of genetic information.
In summary, through the analysis of the ubiquitin and SUMO pathways, the characterization of replisome composition and the molecular dissection of DNA replication, we aim at characterizing the replisome as a dynamic complex that integrates multiple stimuli to ensure genomic stability and contribute to determine cell identity.
Group members

Emilio Lecona Sagrado
Lab.: 409 Ext.: 4736
elecona(at)cbm.csic.es

Rodrigo Martín Rufo
Lab.: 409 Ext.: 4486
rmartin(at)cbm.csic.es

Alba Gil Plaza
Lab.: 409 Ext.: 4486
alba.gil(at)cbm.csic.es

Ana Deodora de la Fuente González
Lab.: 409 Ext.: 4486
a.fuente(at)cbm.csic.es

Alicia Gómez Moya
Lab.: 409 Ext.: 4486
a.gomez.moya(at)cbm.csic.es

Scott Brian Churcher
Lab.: 409 Ext.: 4486
sbchurcher(at)cbm.csic.es

Laura Chuanyang Navarro Leiva
Lab.: 409 Ext.: 4736
Selected publications

Ubiquitin and SUMO as timers during DNA replication
Rodrigo Martín-Rufo et al.

USP7 and VCPFAF1 define the SUMO/Ubiquitin landscape at the DNA replication fork
André Franz et al.

USP7 limits CDK1 activity throughout the cell cycle
Antonio Galarreta et al.

USP7 is a SUMO deubiquitinase essential for DNA replication
Emilio Lecona et al.





