Scientific Program
Interactions with the environment
RESEARCH GROUP
Cellular plasticity in development and cancer

César Cobaleda Hernández
How is the identity of blood cells determined? When their identity is disrupted, cells can become tumoral (leukemia) or non-functional (immunodeficiencies), so understanding cell plasticity is essential for the development of therapies. We study how hematopoiesis is altered in disease using samples from human patients, and mice genetically modified to recapitulate human hematologic diseases.
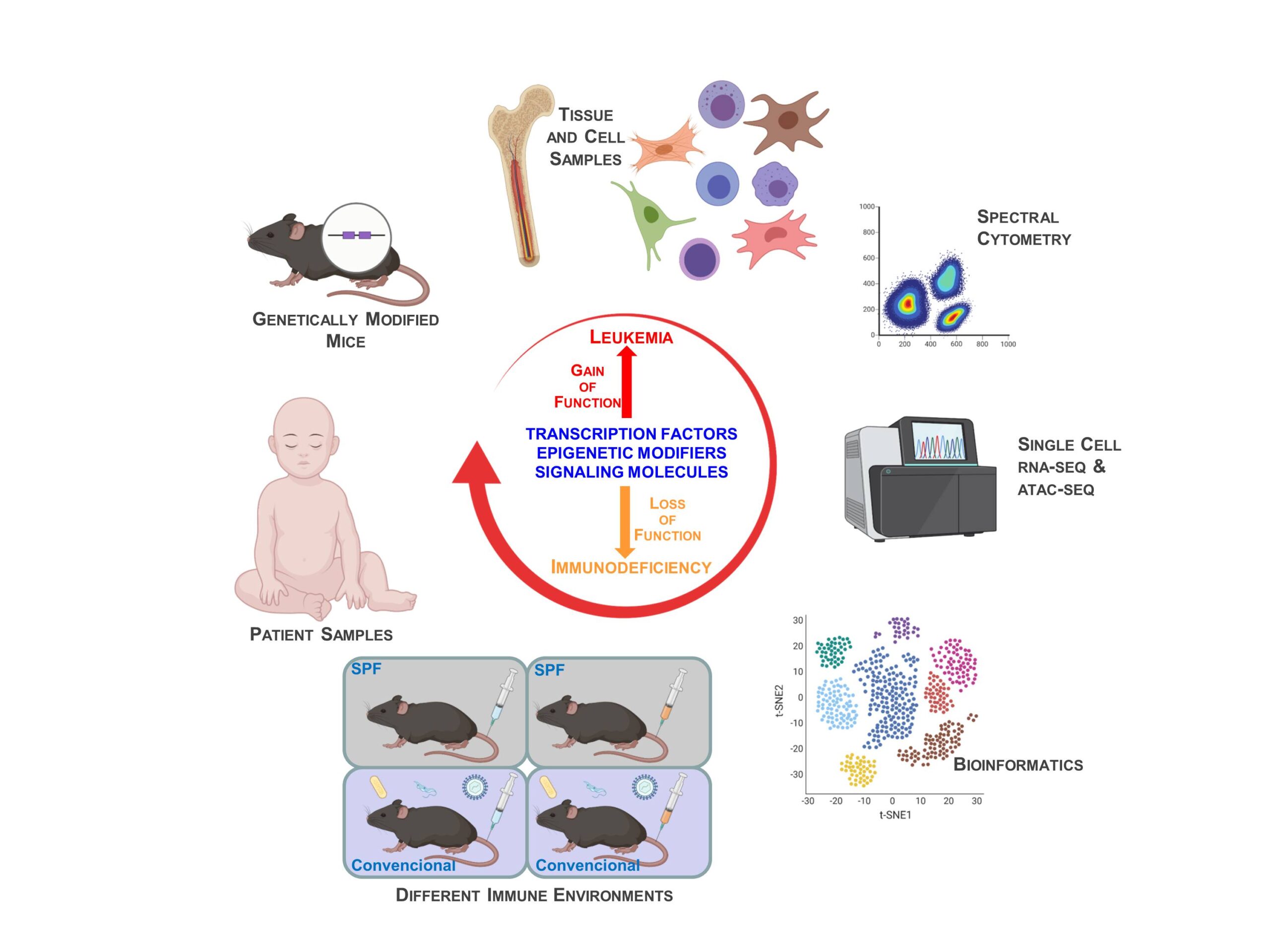
Research
Cellular plasticity, simply put, is the ability of cells to change their identity and convert into a different cell type. It implies the capacity of adopting the gene expression profile of other types of cells and acquiring their functions, and it is an essential characteristic controlled by transcription factors and epigenetic regulators that allow the cells to change their identity by activating or closing down developmental programs. Any alteration of this regulation leads to a pathologic modulation of cellular plasticity, and it usually leads to the appearance of aberrant cellular identities, as is the case in cancer or in developmental genetic syndromes.
Our group wants to understand how cellular identity is determined in the differentiation of lymphocytes, and how it is deregulated in pathological conditions such as leukemias and immunodeficiencies. As experimental tools we use: i) samples from patients and ii) genetically engineered mouse models in which we modify the expression of transcription factors and epigenetic regulators, either normal or oncogenic.
CELLULAR PLASTICITY IN LEUKEMIA
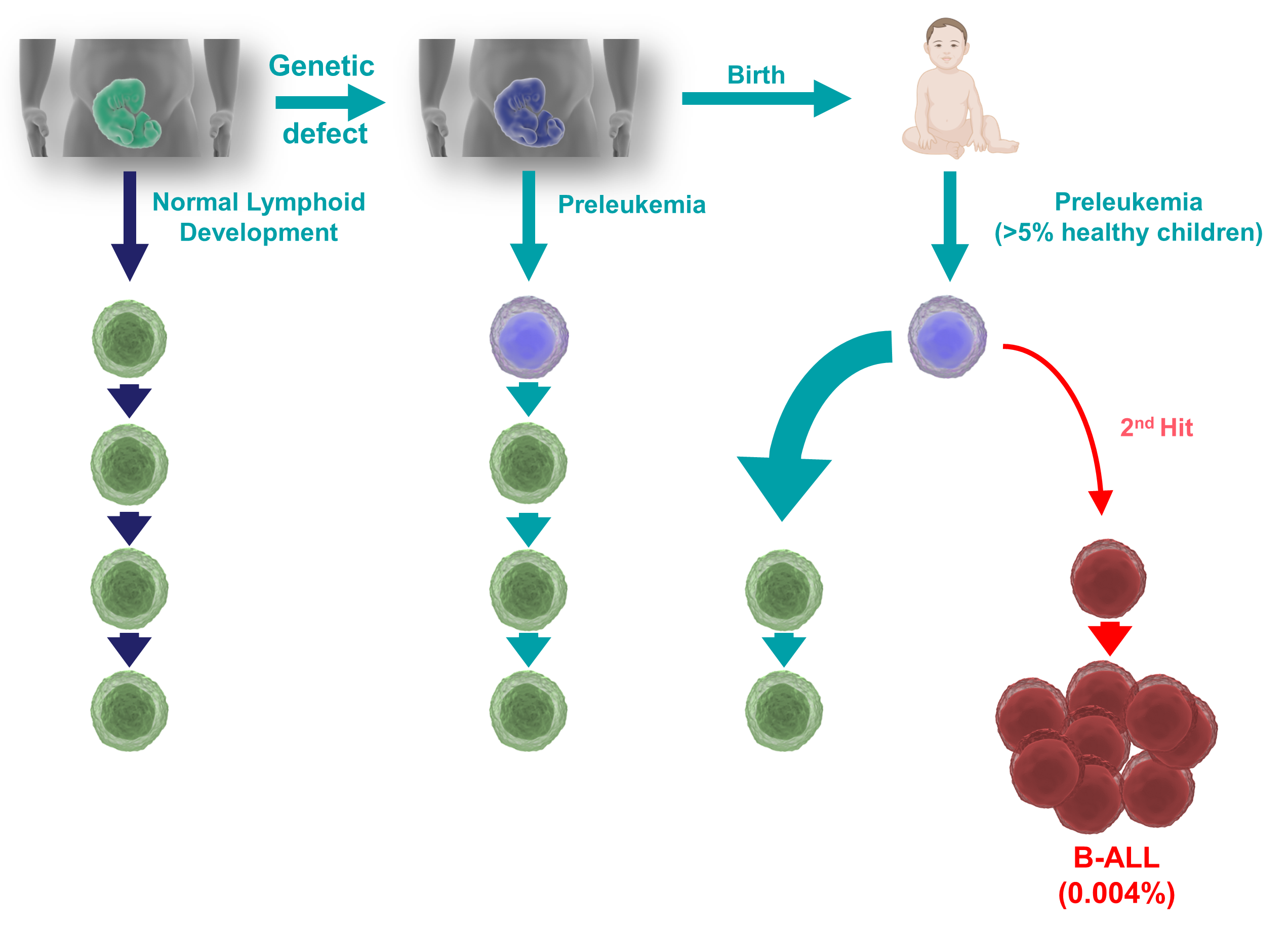
B-cell acute lymphoblastic leukemia (B-ALL) is the most common form of childhood cancer. Interestingly, almost 5% of all newborn children present some form of congenital genetic predisposition to develop B-ALL; luckily, very few (<1%) of these predisposed children will progress to B-ALL, by suffering a second hit that leads to full-blown disease. The causes that trigger this progression are still unclear, but B-ALL incidence seems to be increasing in parallel with the adoption of modern lifestyles. A stress on the immune system has been postulated to be involved in the progression to B-ALL, and this stress could be triggered by exposure to common infections (under certain circumstances), or by other stressors like antibiotics, diet, or alterations in the microbiota. Therefore, understanding the interaction between the immune stress and the preleukemic cells existing in so many newborns could provide us with strategies aimed at preventing childhood B-ALL.
We have generated mice expressing the oncogenic lesions responsible for the initiation of childhood B-ALLs, and we have demonstrated that common infections are indeed one of the main triggering factors leading to leukemia development. Furthermore, we have shown that preventive pharmacological treatment of predisposed mice with inhibitors of oncogenic signaling pathways protects them from the appearance of the disease, showing that B-ALL could be a preventable disease. Since the presence of latent premalignant cells is a common characteristic of many types of cancers, our results offer a general proof-of-principle for the development of similar preventive strategies for other malignancies.
CELLULAR PLASTICITY IN IMMUNODEFICIENCIES
Wolf Hirschhorn Syndrome (WHS) is a complex rare disease caused by the loss of genetic material in the “p” arm of chromosome 4, and affected patients present many severe problems, including life-threatening immunodeficiencies. One of the genes affected in WHS is WHS-Candidate-1 (WHSC1), an epigenetic regulator involved in many processes affecting genome function and that, interestingly, is also mutated in childhood B-ALLs and other malignancies of B-cell origin like Multiple Myeloma (where it is called “Multiple Myeloma SET domain protein”, MMSET). We are using genetically engineered mouse models with deregulated Whsc1 function to gain insight into the molecular biology of WHS-associated hematopoietic pathologies, in order to better understand the role of epigenetics in normal and aberrant hematopoietic development, and to develop future prospective therapeutic interventions. We have shown that the loss of Whsc1 is essential for the normal differentiation and function of B cells, therefore experimentally linking it to the immunodeficiency of WHS patients, and generating a preclinical model for therapy development.
CELLULAR PLASTICITY IN MULTIPLE MYELOMA
We have collaborated in the generation of new MMSET-based multiple myeloma mouse models recapitulating the characteristics of the human disease and showing a correlation between the genetic and immunological characteristics of each tumor and its response to preclinical therapies, therefore enabling the prediction of treatment outcomes with next-generation immunotherapies.
Group members

César Cobaleda Hernández
Lab.: 325 Ext.: 4692
cesar.cobaleda(at)csic.es

Rodrigo Bailey Maldonado
Lab.: 325 Ext.: 4469
rbailey(at)cbm.csic.es

Laura Moreno de Lara
Lab.: 325 Ext.: 4691
lmoreno(at)cbm.csic.es

Raquel Cantalejo Gómez
Lab.: 325 Ext.: 4692
Selected publications
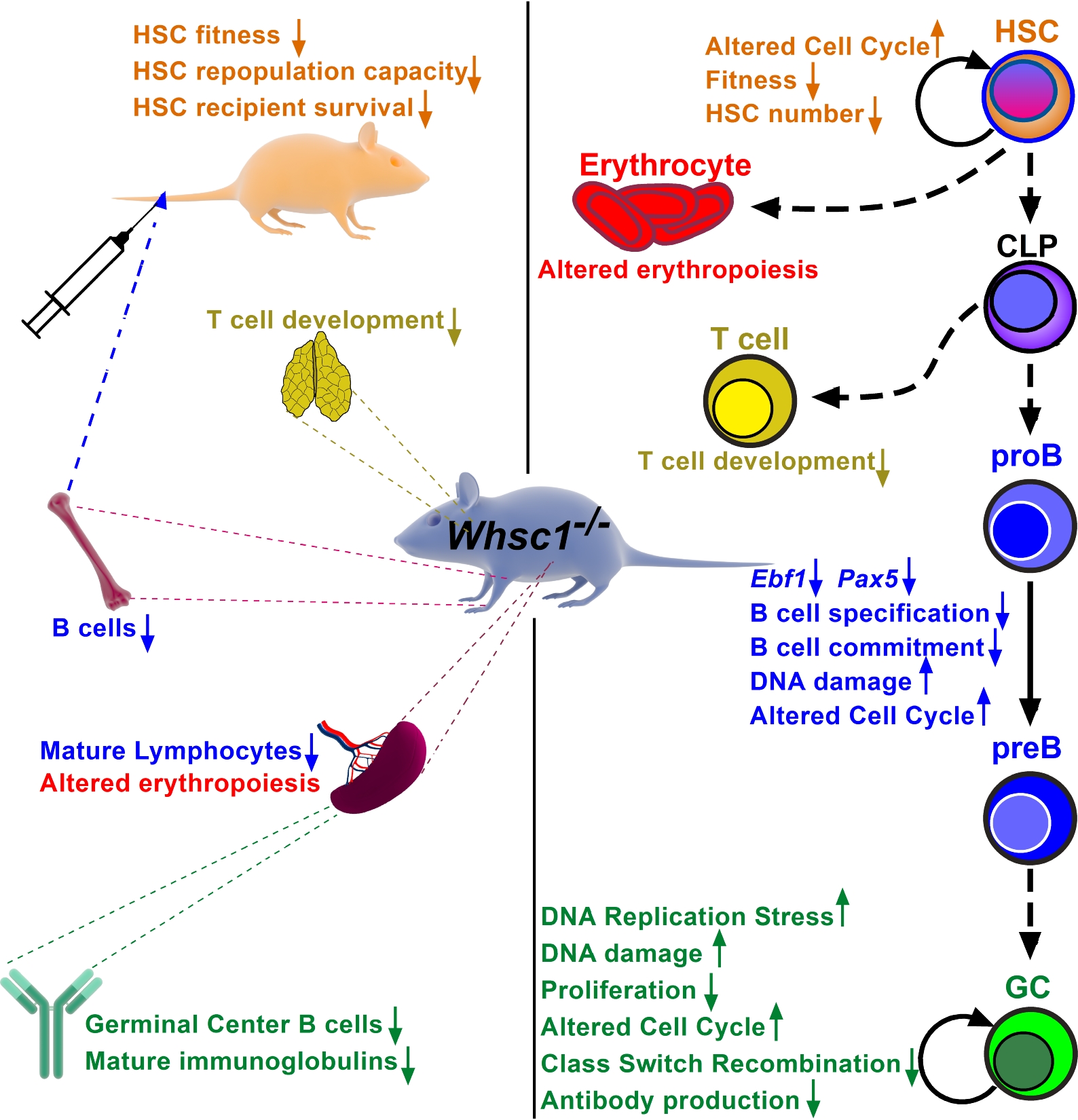
Wolf-Hirschhorn Syndrome Candidate 1 Is Necessary for Correct Hematopoietic and B Cell Development
Elena Campos-Sanchez et al.
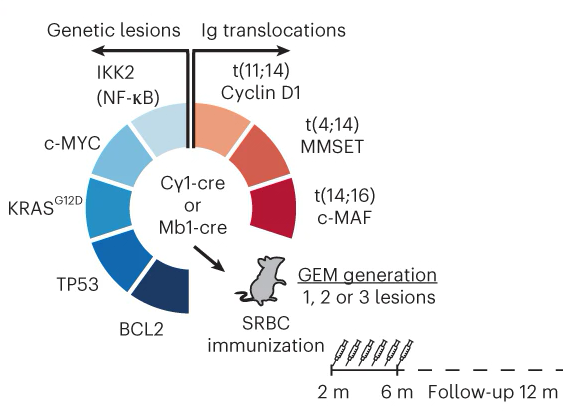
Preclinical models for prediction of immunotherapy outcomes and immune evasion mechanisms in genetically heterogeneous multiple myeloma
Marta Larrayoz et al.
Infectious triggers and novel therapeutic opportunities in childhood B cell leukaemia
Cesar Cobaleda et al.
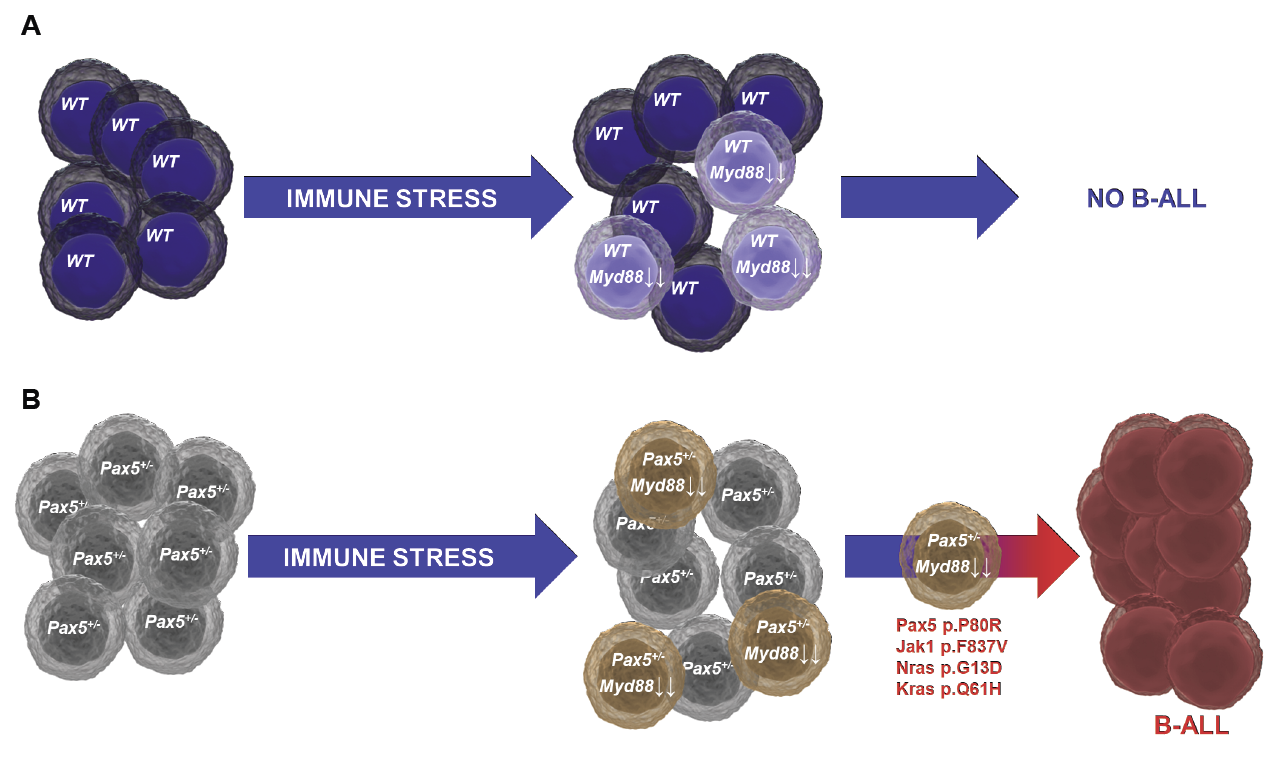
Immune stress suppresses innate immune signaling in preleukemic precursor B-cells to provoke leukemia in predisposed mice
Marta Isidro-Hernández et al.




