Scientific Program
Interactions with the environment
RESEARCH GROUP
TCR domains in T cell differentiation and (patho)physiological and therapeutic responses

Hisse M van Santen
We study in cellular and animal models the mechanisms via which T cell Receptors achieve high sensitivity for antigen, how they are able to generate signals of different strength and how this impacts on protective and auto-immune responses. We also apply this knowledge to development and optimization of recombinant immune receptors that can be used for cellular immunotherapy against cancer.
Research
T cells orchestrate the immune response against pathogens such as viruses and bacteria. Each T cell expresses a unique T cell receptor (TCR) that is generated by a random gene assembly process, giving rise to a hugely diverse repertoire of TCRs that at the population level can recognize peptides derived from virtually any pathogen. Activation of the T cell upon TCR-mediated recognition of pathogen-derived peptides gives rise to beneficial immune responses, while responses against peptides derived from the host itself can lead to auto-immune manifestations. We aim to understand how TCRs signal, focusing on how their organization on the cell membrane favors their sensitivity for antignic peptides. We use transgenic mouse models to study the role of TCR nanoclusters during early T cell differentiation, where a TCR signaling strength-based selection process takes place that filters out potentially autoimmune T cells, and to understand how this organization impacts on the ability to mount protective memory and undesired autoimmune responses.
We also develop approaches to improve T cell responses against cancer, focusing on the signaling domains of recombinant tumor-specific receptors. Our research shows that the identity and topology of TCR-derived signaling domains of these recombinant receptors determines their efficiency with respect to activating the T cells. These results have a direct implication for improving cellular cancer immunotherapy. They also provide new inroads into understanding the mechanisms underlying TCR function.
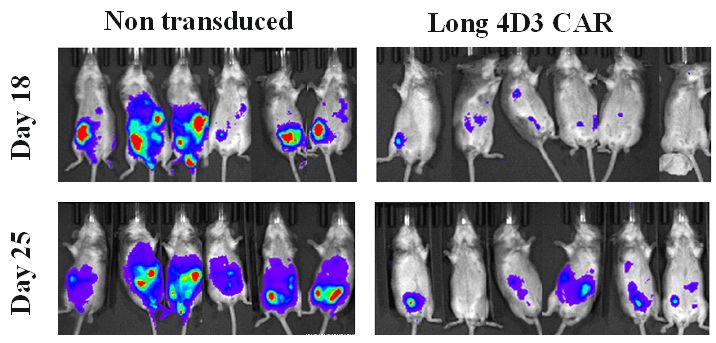
In a third, clinically-oriented project, we have generated CAR-T cells against Acute Myeloid Leukemia (AML). While CAR-T cells that can recognize various types of B cell lymphoma and leukemia have been very successful in the clinic, use of CAR-T cell therapy for AML treatment has been severely hampered by unacceptable toxicity against the patient’s myeloid precursors that express the same antigen as the ones targeted on the AMLs. As no better target antigens have been defined, we are generating CAR-T cells that use a logic gate formed by an activating CAR recognizing the shared antigen on precursors and AMLs and an inhibitory immune receptor that recognizes a ligand only expressed by the myeloid precursors. If successful, this concept can be applied to other type of cancers using appropriate combinations of activating and inhibiting receptors.
Group members

Hisse Martien Van Santen
Lab.: 222 Ext.: 4682
hvansanten(at)cbm.csic.es

Irene Serrano Pérez
Lab.: 222 Ext.: 4682
iserrano(at)cbm.csic.es

Gala Álvarez Neila
Lab.: 222 Ext.: 4682
Selected publications

A set point in the selection of the αβTCR T cell repertoire imposed by pre-TCR signaling strength
Elena R. Bovolenta et al.

Cognate peptide-MHC complexes are expressed as tightly apposed nanoclusters in virus-infected cells to allow TCR crosslinking
María Ferez et al.

Increased sensitivity of antigen-experienced T cells through the enrichment of oligomeric T cell receptor complexes
Rashmi Kumar et al.
Title
Authors





