Scientific Program
Interactions with the environment
RESEARCH GROUP
Unconventional autophagy in health and disease

Felipe X. Pimentel-Muiños
We study how the molecular machinery that regulates autophagy also participates in a variety of subcellular processes apparently unrelated to the classical autophagic process that degrades cytoplasmic components. We use molecular and cellular biology approaches, and also animal models, to characterize these novel processes, evaluate to what extent their dysfunction causes disease, and assess their possible value as therapeutic targets in a variety of pathologies.
Research
Autophagy is a degradative process that eliminates obsolete or potentially damaging cytoplasmic components and is critical for cellular homeostasis. Judging from the pathological phenotypes observed in mice lacking the expression of proteins that constitute the autophagic machinery (the so-called ATGs), autophagy has an important role in protection against a variety of pathologies, including cancer, inflammatory diseases or some neurodegenerative disorders. This protective capacity suggests that manipulation of autophagy could hold therapeutic value, a possibility that is being intensely studied by a variety of laboratories around the world. However, it is nowadays becoming clear that the different ATGs also have alternative functions not strictly related to the canonical autophagic pathway, either because their final purpose is not degradative, or because the relevant ATGs, even promoting catabolic outcomes, function in unconventional ways that are fundamentally different from autophagy. The contribution of these alternative activities to preventing the pathological phenotypes caused by ATG elimination is currently unclear, but their characterization is an important issue because it could determine the way ATG activity could be manipulated with therapeutic purposes.
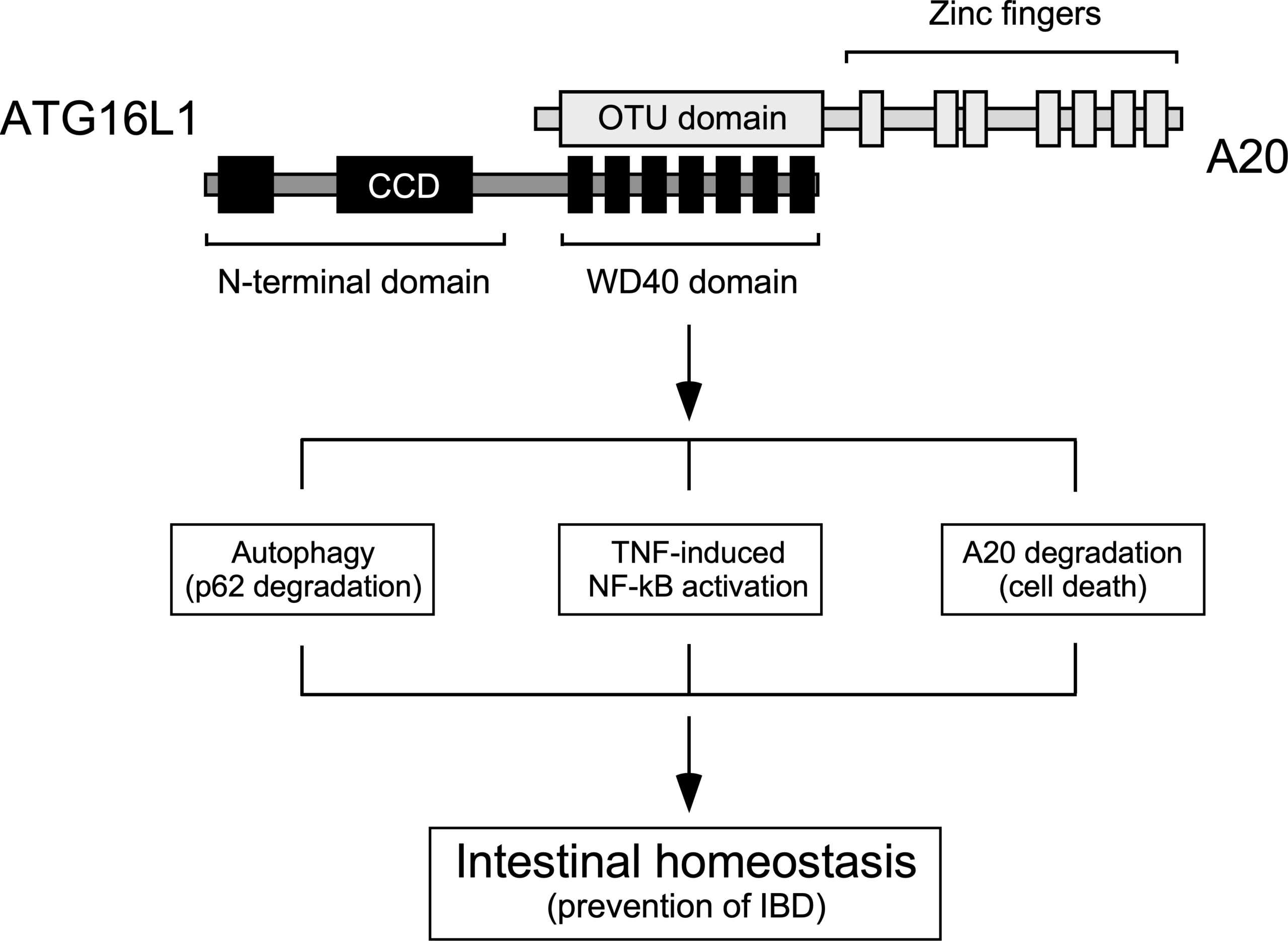
With this overarching goal in mind, our lab focuses on ATG16L1 as a paradigm to discover and characterize new unconventional activities of the autophagic machinery. In its most canonical function, ATG16L1 is critical for proper formation of autophagosomes, double-membrane vesicles that sequester the cellular material that will eventually be degraded in the lysosome through the autophagic route. However, ATG16L1 includes a C-terminal domain that is irrelevant for this activity and seems to play unconventional roles in a wide variety of cellular processes. Projects currently being developed in the lab intend to discover and characterize in detail novel unconventional functions of ATG16L1 and other members of the general autophagic machinery, and explore how they interact with the conventional pathway. Current areas of interest in the lab include:
Regulation of endocytosis, intracellular trafficking and signaling output of cytokine receptors
Control of the stability of signaling mediators and the regulation of inflammatory responses
Regulation of immunogenic cell death and its impact in cancer and response to chemotherapy
Innate immunity against intracellular bacterial infections
To characterize these processes we use a wide variety of experimental systems include in vitro (cell lines) as well as in vivo (animal models) genetically engineered using the CRISPR/Cas9 system, proteomics techniques and bioinformatics approaches to identify novel mediators. We are currently trying to characterize in depth these mechanisms, discover novel non-canonical activators of ATG16L1 and explore how all these activities may be influenced by a coding polymorphic allele of ATG16L1 (T300A) whose presence in homozygosis increases the risk of suffering different pathologies, including Crohn’s disease, a serious intestinal inflammatory disease that is currently incurable. In this particular context, we intend to explore the molecular bases of complex, multifactorial diseases caused by the T300A allele, like Crohn’s disease. Exploring and establishing ways to manipulate atypical autophagy with therapeutic intentions is also a central long-term goal of our group that we intend to develop over the next few years.
Group members

Tamara Rosell García
Lab.: 411 Ext.: 4737
trosell(at)cbm.csic.es

Felipe X. Pimentel Muiños
Lab.: 411 Ext.: 4737
fxp(at)cbm.csic.es

Daniel Antonio Oña Sánchez
Lab.: 411 Ext.: 4738
donia(at)cbm.csic.es

Julia Bandera Linero
Lab.: 411 Ext.: 4738
julia.bandera(at)cbm.csic.es

Santiago Tomás Díaz Neto
Lab.: 411 Ext.: 4738
santiagodiazneto(at)cbm.csic.es

Rocío Díaz Parra
Lab.: 411 Ext.: 4738
rocio.diaz(at)cbm.csic.es
Selected publications
Regulation of cytokine signaling through direct interaction between cytokine receptors and the ATG16L1 WD40 domain
Inmaculada Serramito-Gómez et al.
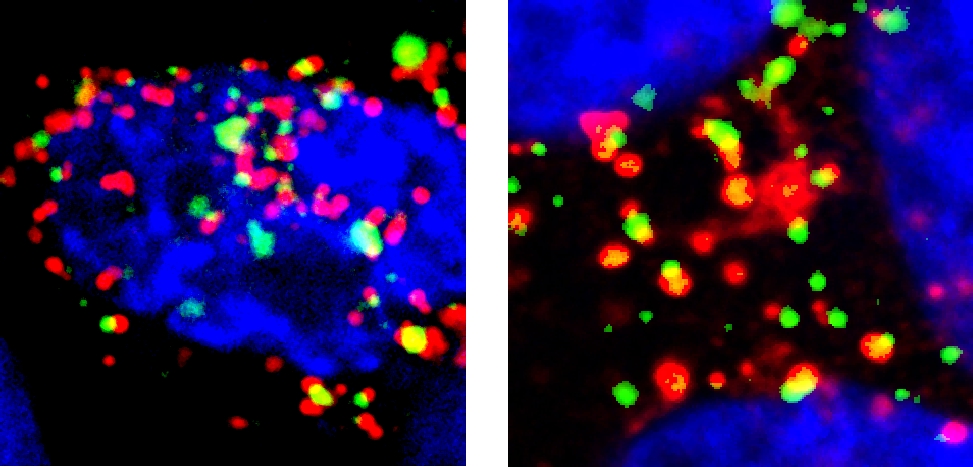
Physical and functional interaction between A20 and ATG16L1-WD40 domain in the control of intestinal homeostasis
Karolina Slowicka et al.
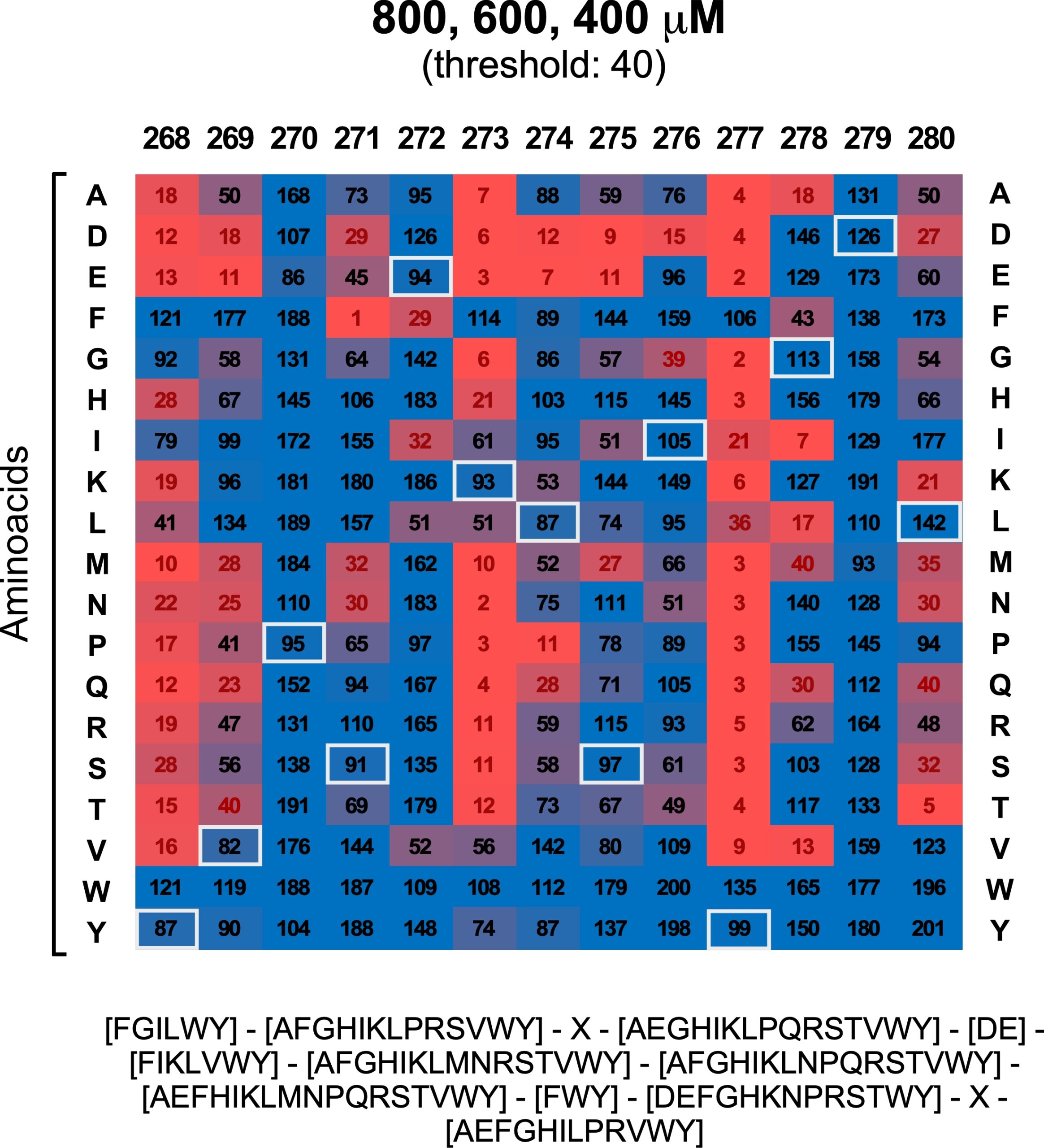
The T300A Crohn’s disease risk polymorphism impairs function of the WD40 domain of ATG16L1
Emilio Boada-Romero et al.
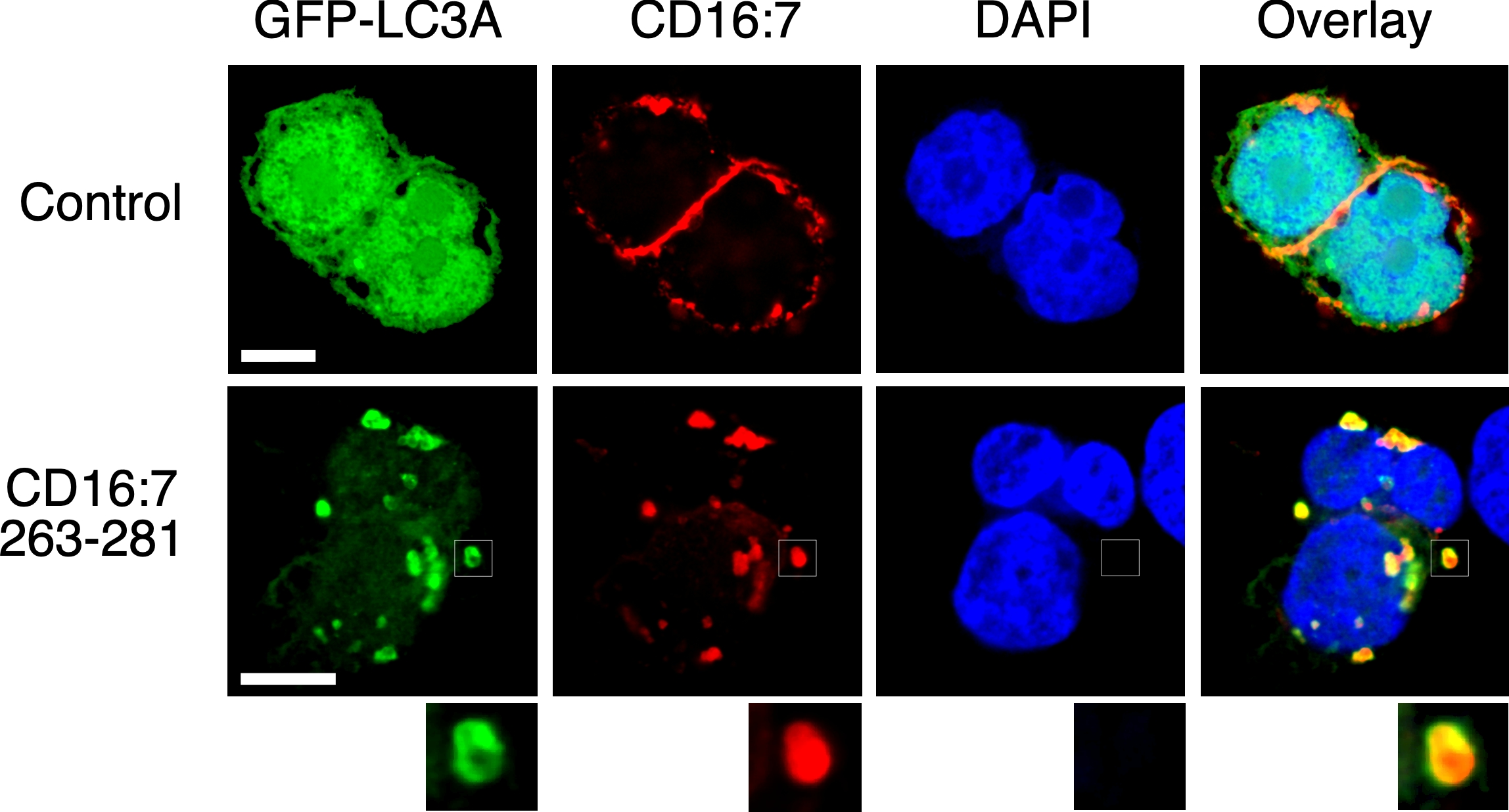
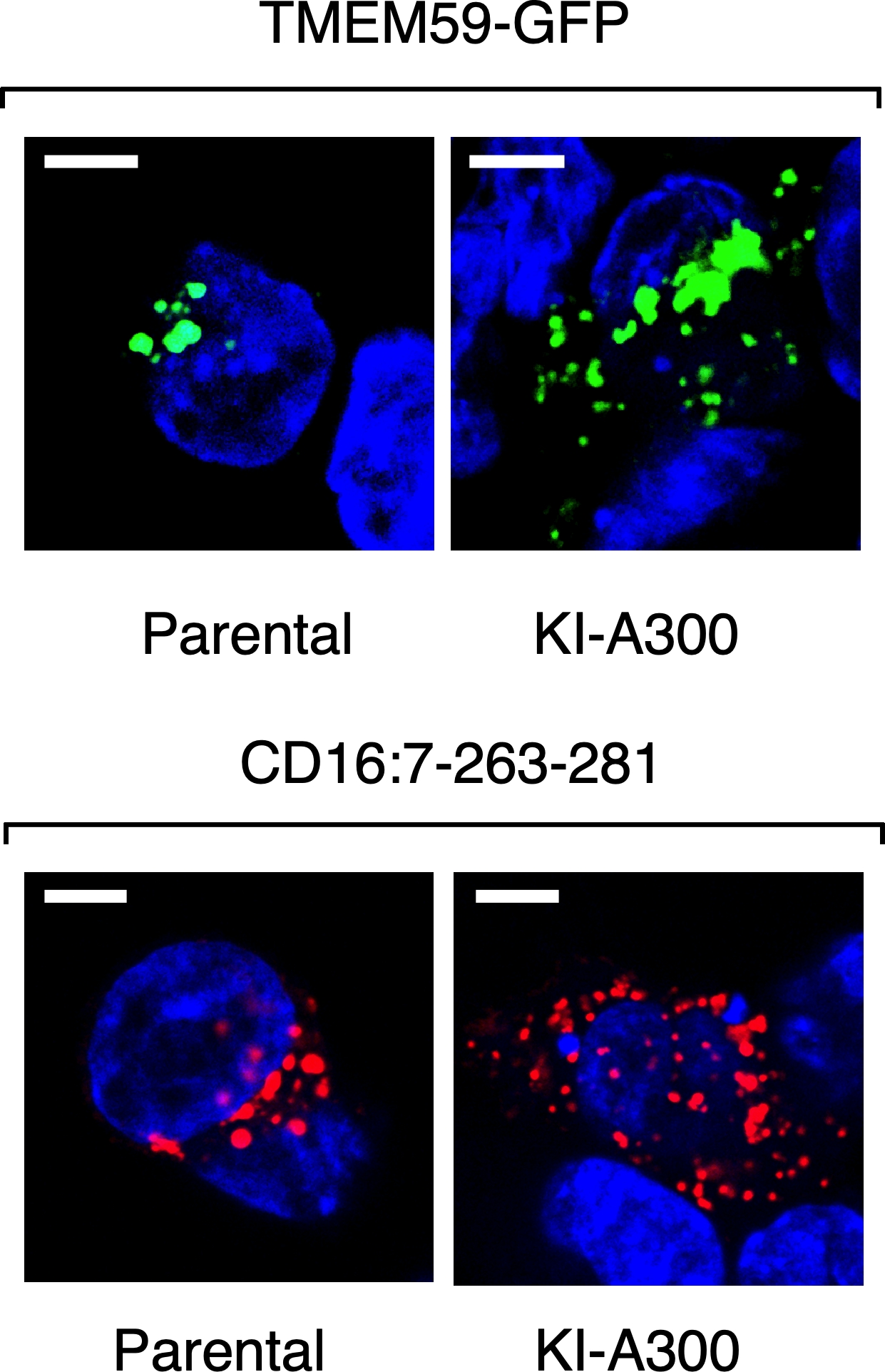
TMEM59 defines a novel ATG16L1-binding motif that promotes local activation of LC3
Emilio Boada-Romero et al.





