Scientific Program
Interactions with the environment
RESEARCH GROUP
Human immunodeficiency virus reverse transcriptase and antiretroviral therapy

Luis Menéndez Arias
Our current research in human immunodeficiency virus (HIV) replication is focused in the discovery of new antiviral drugs acting on targets unexploited in current therapies (i.e. ribonuclease H), and understanding reverse transcriptase function and activity to improve its biotechnological potential.
Research
Our current research in HIV replication is focused in the discovery of new antiviral drugs acting on targets unexploited in current therapies (i.e. ribonuclease H), and understanding reverse transcriptase (RT) function and activity to improve its biotechnological potential.
In collaboration with Chinese researchers we have recently described novel double-winged galloyl derivatives and a series of coumarin-based compounds as potent HIV-1 RNase H inhibitors (IC50 values around 65 nM and 4 µM, respectively). Selected coumarin derivatives were characterized as dual inhibitors of HIV-1 DNA polymerase and RNase H activities, and showed antiviral efficiency in cell culture (EC50 ~ 5.6 µM). Recently, we demonstrated that mutational inactivation of the RT’s RNase H eliminates its strand transfer efficiency while diminishing RNA-dependent strand displacement activity. Interestingly, the residual activity can be completely eliminated by adding coumarin derivatives and this property could be used in biotechnological applications to obtain more uniform read coverages when copying long RNAs, particularly when using several primers.
Current projects include: (1) Searching for novel RNase H inhibitor candidates against HIV-1 and HIV-2 major clades, and its extension to other viral targets (e.g. hepatitis B virus and herpesviruses), based on the conservation of the RNase H-fold in viral enzymes (e.g. HIV-1 integrase, exonucleases, etc.), and (2) designing novel RTs with increased affinity for RNA/DNA complexes in order to improve the cDNA synthesis efficiency in the presence of minute amounts of RNA. We will use rational design to obtain engineered enzymes with higher nucleic acid binding affinity, and in addition, we plan to obtain fusion proteins containing domains that hopefully will improve the RT’s catalytic efficiency (e,g, viral NC, topoisomerase, and helicases). In addition, we plan to develop methods to assess fidelity of DNA synthesis in an effective and inexpensive manner and to study the molecular determinants controlling this property.
Group members

Luis Menéndez Arias
Lab.: 124 Ext.: 4494
lmenendez(at)cbm.csic.es

Sabina Andreu Satue
Lab.: 124 Ext.: 4523
sandreu(at)cbm.csic.es

María de la Vega Craqui Cordero
Lab.: 124 Ext.: 4494

Maialen Crespo Rodríguez
Lab.: 124 Ext.: 4494

Lukas Gosmann
Lab.: 124 Ext.: 4523/4474
Selected publications
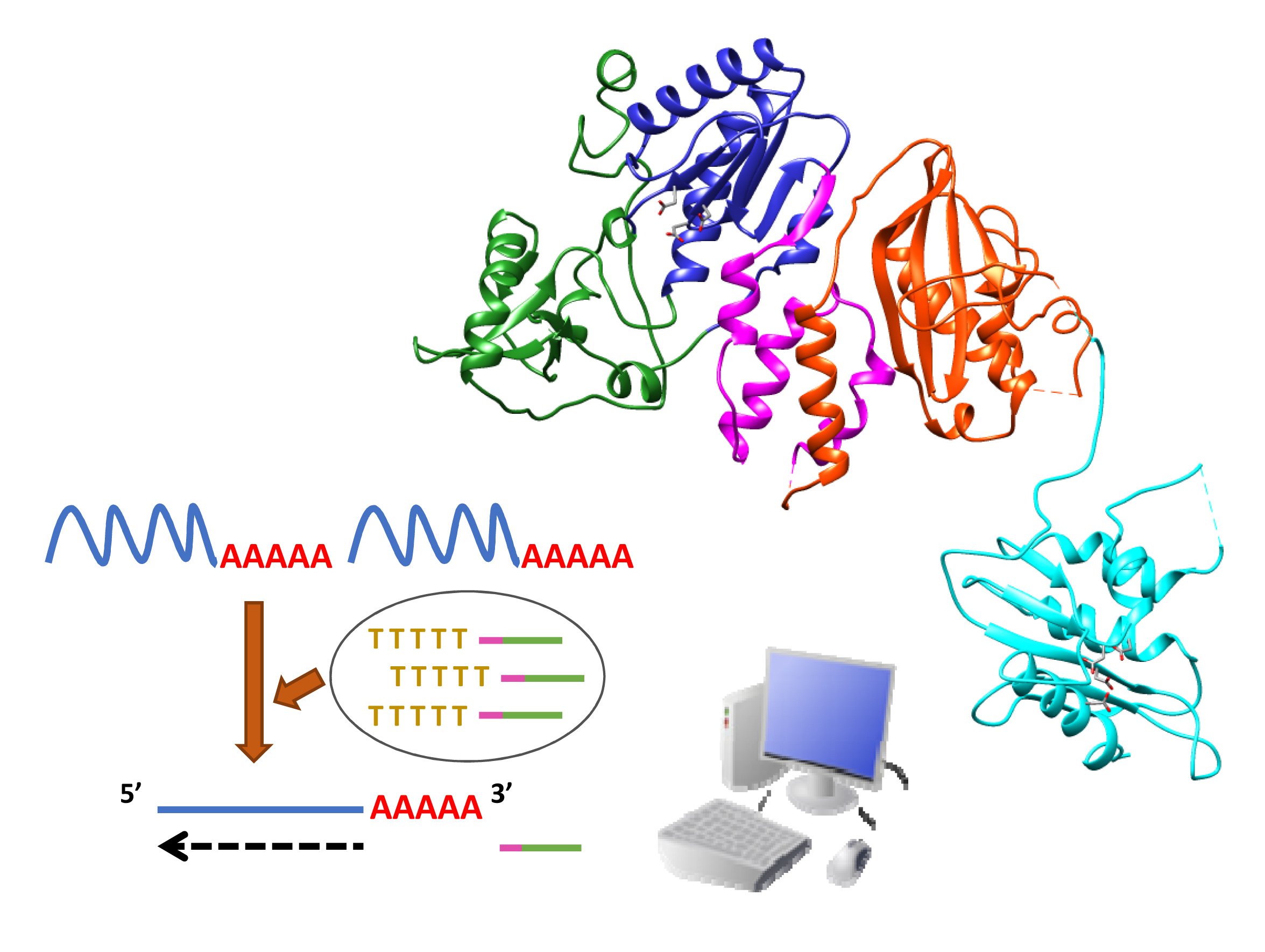
Reverse Transcriptase: From Transcriptomics to Genome Editing
Samara Martín-Alonso et al.
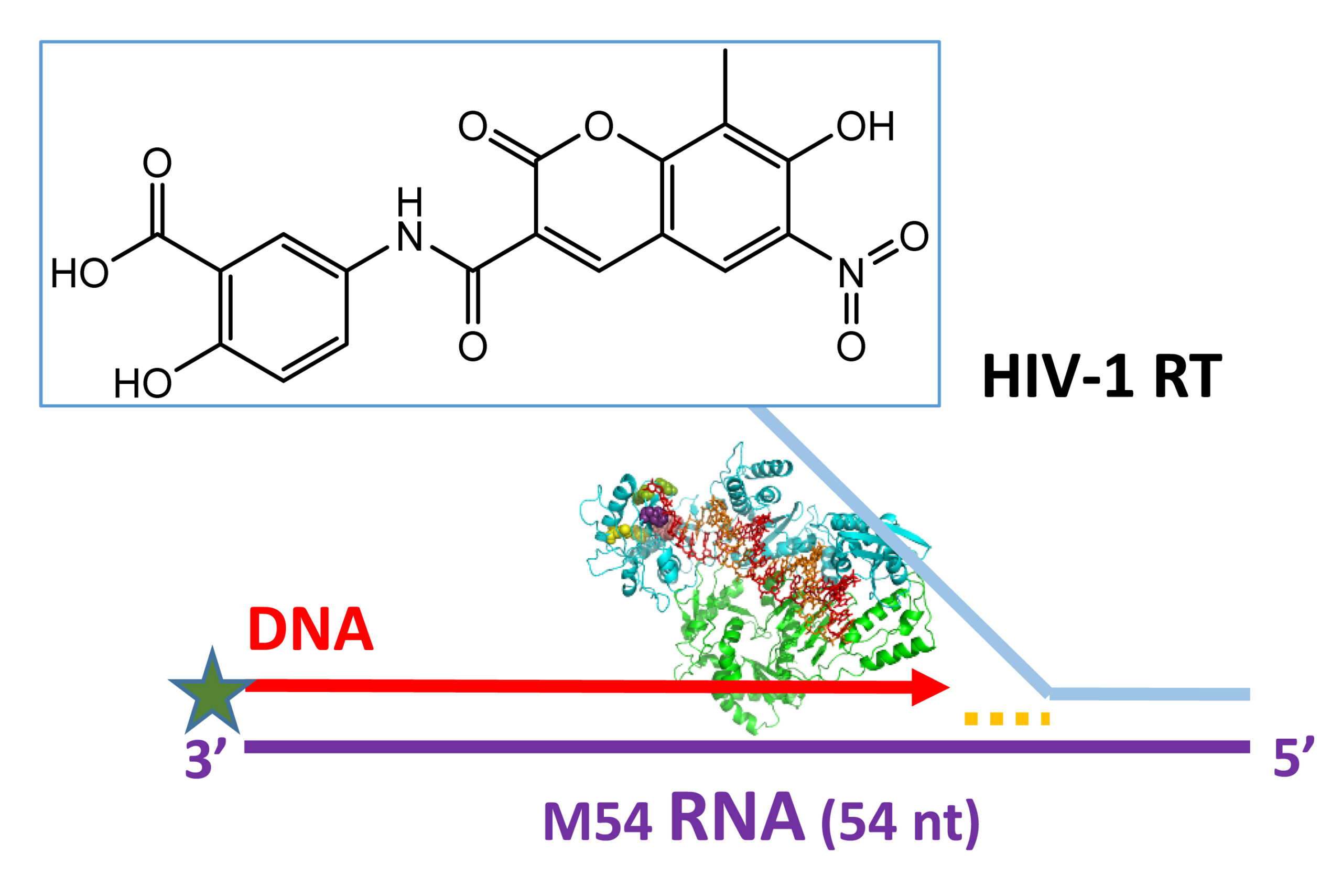
Novel RNase H Inhibitors Blocking RNA-directed Strand Displacement DNA Synthesis by HIV-1 Reverse Transcriptase
Samara Martín-Alonso et al.
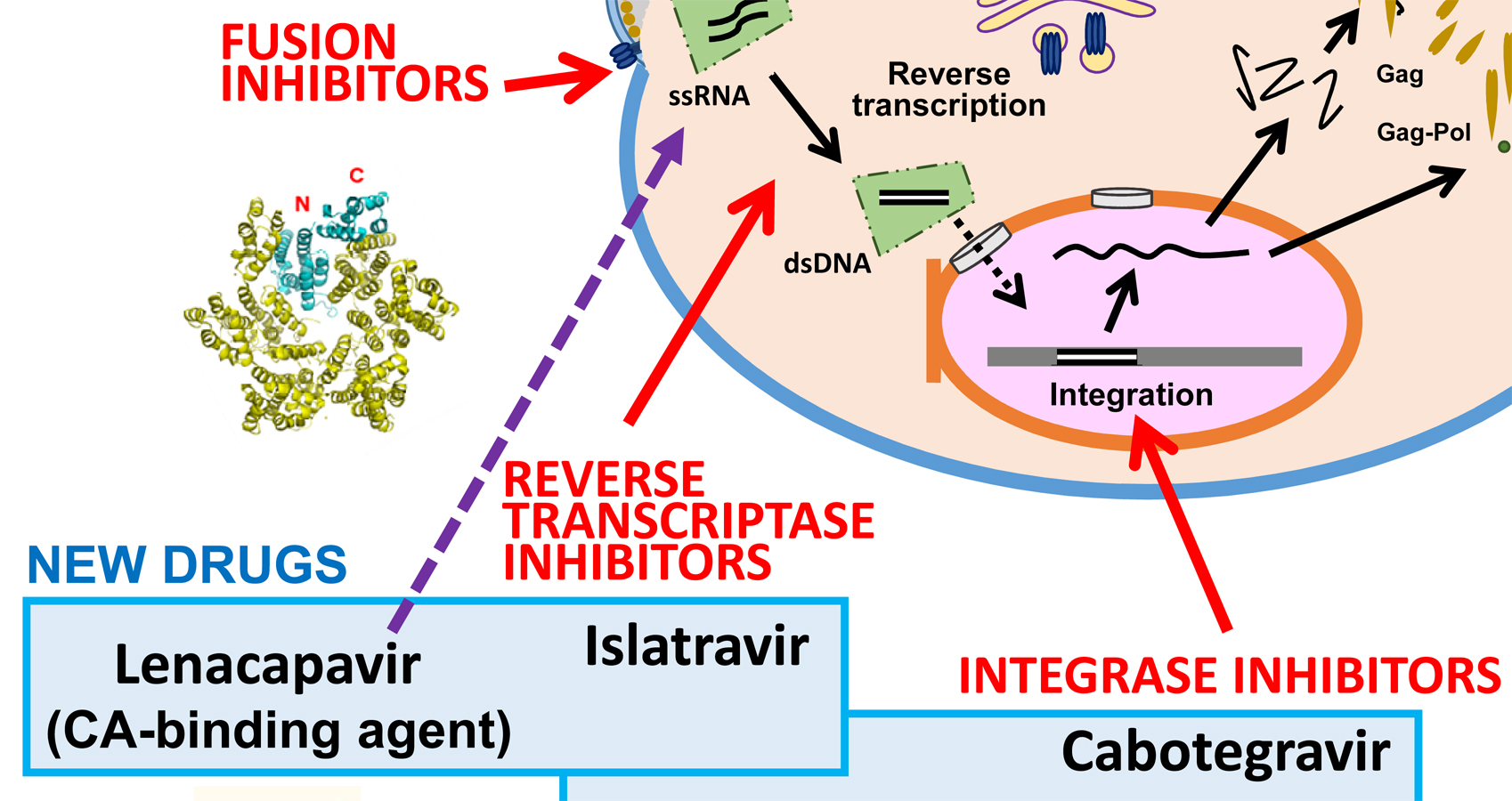
Update and latest advances in antiretroviral therapy
Luis Menéndez-Arias et al.
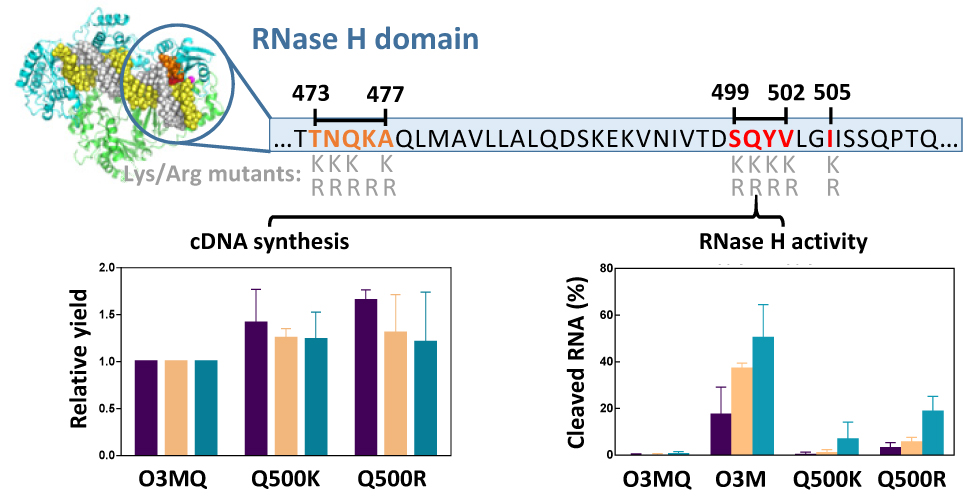
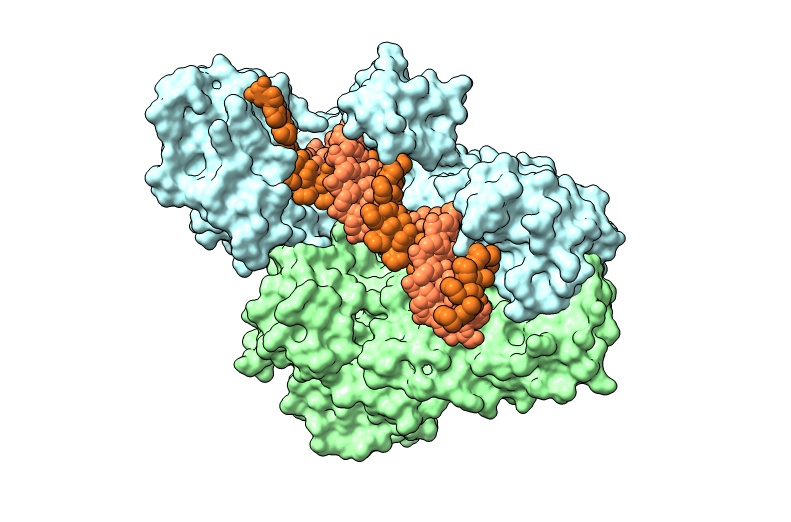
Charge Engineering of the Nucleic Acid Binding Cleft of a Thermostable HIV-1 Reverse Transcriptase Reveals Key Interactions and a Novel Mechanism of RNase H Inactivation
Javier Martínez Del Río et al.