Scientific Program
Interactions with the environment
RESEARCH GROUP
Yeast enzymes bioengineering to generate bioactive compounds

Maria Fernandez Lobato
We work with microorganisms producing bioactive compounds and try to connect the generation of knowledge to the development of biotechnological applications. We focus on characterization of enzymes producing new compounds, analysis of their structural-functional determinants, their operational improvement, characterization of the molecules produced and evaluation of their potential biological activity.
Research
We work with microorganisms of biotechnological interest, mainly fungi and yeasts, producers of bioactive compounds. We try to connect the generation of knowledge to the development of biotechnological applications. Basically we focus on the characterization of new enzymes producing bioactive compounds, the analysis of their structural-functional determinants, the operational improvement using molecular biology tools and in obtaining and characterization of new molecules with potential industrial utility. We have patented in different countries the industrial applicability of most proteins characterized and designed methods for their attachment to solid supports.
During the last years we have been characterizing and studying several fungi and non-conventional yeast proteins (from genera Xanthophyllomyces, Schwanniomyces, Rhodotorula, etc.) showing glycosyltransferase activity, and applicable in the production of sugars with prebiotic properties. All are glycosylhydrolases (GH) structurally included in family GH32, 31, 13 or 18. Indeed, we have resolved the 3-D structure of the first yeast protein including in family GH32, assigned a function to the beta-sandwich domain that is present in all members of this family and proved that the oligomerization is directly involved in the substrate recognition and specificity. We have obtained numerous enzymatic variants that increase or alter the biosynthetic product patterns. Recently we have found that some of the characterized enzymes can glycosylate compounds with aromatic rings such as the hydroxytyrosol or pterostilbene (both antioxidants), which confers them a special biotechnological interest. We intend to extend our study to hydrolases including in other structural families, to increase and modify the transferase/biosynthetic activity of the enzymes studied, to scale up to industrial level the enzyme production and the products generated, as well as to validate the biological activity or give new uses to the molecules obtained. Objectives included in those of the consortia Glicoenz, Fish4Fish and a project founded by the Fundación Ramon Areces (XIX Concurso Nacional-Ciencias de la Vida y la Materia).
Group members

Miguel Remacha Moreno
Lab.: 102 Ext.: 4521
mremacha(at)cbm.uam.es

María Fernández Lobato
Lab.: 102 Ext.: 4492
mfernandez(at)cbm.csic.es

Marina Minguet Lobato
Lab.: 102 Ext.: 4521
m.minguet(at)cbm.csic.es

Egle Narmontaite
Lab.: 102 Ext.: 4521
egle.narmontaite(at)cbm.csic.es

María Martínez Ranz
Lab.: 102 Ext.: 4521
mmranz(at)cbm.csic.es

Laura Barahona Pérez
Lab.: 102 Ext.: 4521
lbarahona(at)cbm.csic.es

Dana Gabdrashitova
Lab.: 104 Ext.: 4504

Beatriz Gracia Gómez
Lab.: 102 Ext.: 4492
beatriz.gracia(at)cbm.csic.es

Emilio García Canalejas
Lab.: 102 Ext.: 4521

Belén García Lacalle
Lab.: 102 Ext.: 4492
belen.garcia(at)cbm.csic.es

Alessandra Pinedo Puma
Lab.: 102 Ext.: 4492
Selected publications
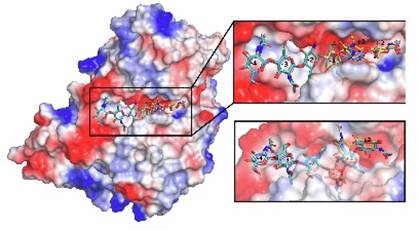
Structural and kinetic analysis of Schwanniomyces occidentalis invertase reveals a new oligomerization pattern and the role of its supplementary domain in substrate binding
Miguel Alvaro-Benito et al.
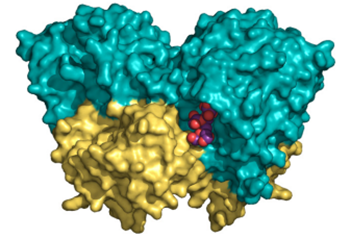
Structural and kinetic insights reveal that the amino acid pair Gln-228/Asn-254 modulates the transfructosylating specificity of Schwanniomyces occidentalis β-fructofuranosidase, an enzyme that produces prebiotics
Miguel Álvaro-Benito et al.
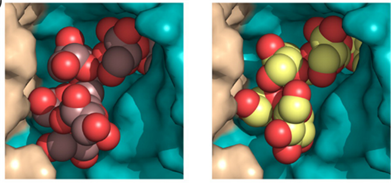
Structural Analysis of β-Fructofuranosidase from Xanthophyllomyces dendrorhous Reveals Unique Features and the Crucial Role of N-Glycosylation in Oligomerization and Activity
Mercedes Ramírez-Escudero et al.
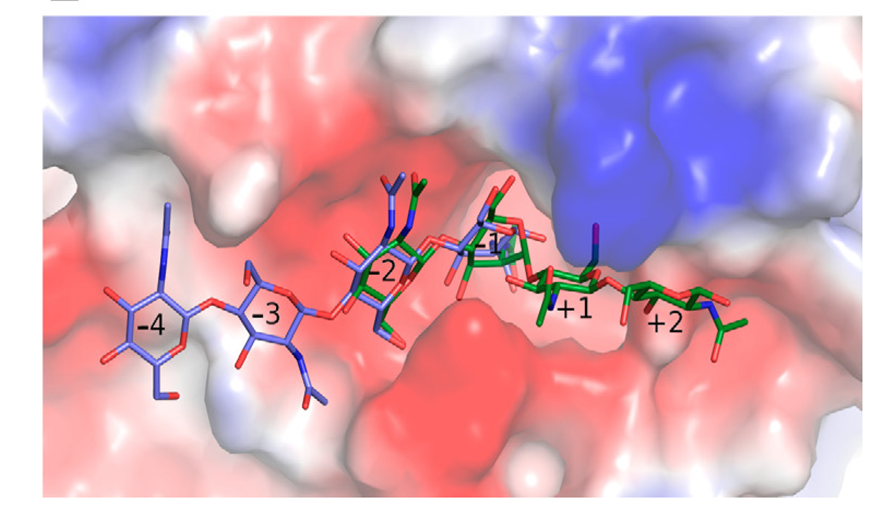
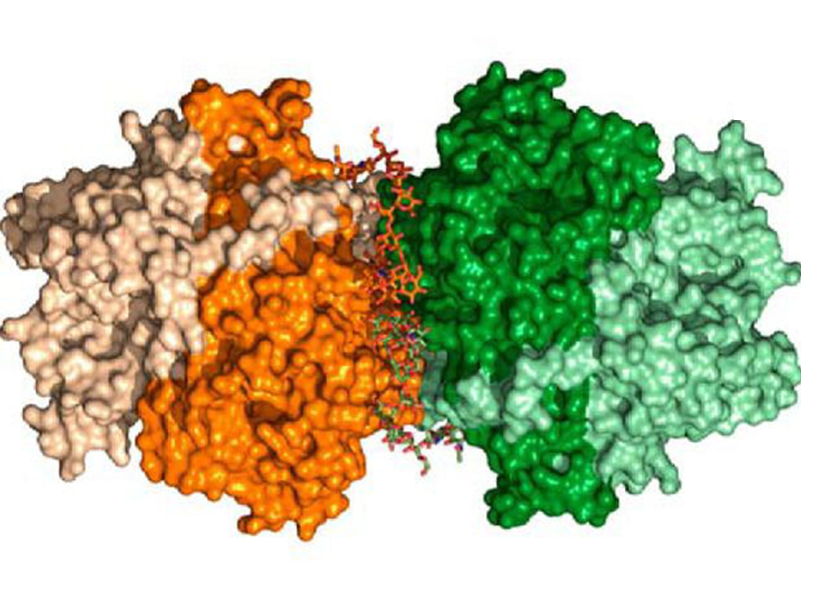
Structure–Function Insights into the Fungal Endo-Chitinase Chit33 Depict its Mechanism on Chitinous Material
Elena Jiménez-Ortega et al.





