Scientific Program
Physiological and pathological processes
RESEARCH GROUP
Cell communication in homeostasis and disease through new Gq-GPCR signaling nodes

Catalina Ribas Núñez
Our group is interested in understanding how cells communicate to each other and their surroundings under physiological and pathological conditions, focusing on two GPCR signaling nodes, Gαq and GRK2. The microenvironment of a cell is an organized combination of extracellular matrix (ECM), stromal tissue (blood vessels, immune cells, fibroblasts and signaling molecules) that influences the cellular phenotype through physical, mechanical, and biochemical mechanisms. We are interested in the interplay among cell types in their specific environment in two different scenarios: (1) Emerging aspects of cancer research include the interaction between tumor cells and their surrounding stroma, and how these cells can alter their metabolism to survive. However, what enables the tumour to integrate both processes to promote malignancy and metastasis? Considering the importante of GPCR in cancer and the novel role of the Gαq protein as a central regulator of the autophagic, our goals will be to investigate the involvement of Gαq as a link between both processes in the context of oral cancer (HNSCC). We will focus on assessing the importance of the GPCR-Gαq pathway in the regulation of tumor-associated stromal architecture by CAFs (tumour-associated fibroblasts), as well as its role as a regulator in the cross-modulation between exosome secretion and the different autophagic flux pathways, both fundamental mechanisms in tumour-stromal communication. (2) Homeostasis also requires intercommunication between different cell types in their tissue environment, often involving cells of the immune system. Proper functional interactions between epidermal, dermal, and immune cell infiltration are necessary for correct homeostasis in stratified epithelia (such as skin or oral tissues). We reported that GRK2 downregulation correlates with tumor malignancy in epithelial Head and Neck Squamous Cell Carcinoma (HNSCC) and increases invasion of oral cancer cells in mice. Considering that GRK2 loss in stratified epithelia induces alterations in epidermal homeostasis, an open raised question is, how is the dialogue between keratinocytes and their cellular microenvironment fine-tuned to facilitate coordinated responses to maintain homeostasis against inflammatory diseases and cancer? To answer these questions, we will use biochemical, cell biology and exosome biology techniques, different “omic” methodologies (transcriptomics, proteomics, metabolomics), high-resolution microscopy and flow cytometry, and 2D and 3D migration/invasion systems, combined with animal models with altered Gαq or GRK2 expression. Our work will contribute to the understanding of the molecular mechanisms preserving epithelial homeostasis and lead to the discovery of novel targets and treatment approaches associated with the development of HNSCC, the sixth most prevalent cancer form in the world with a very poor prognosis and patient survival

Research
Cell-cell communication and the interactions that occur between them are a key aspect in the maintenance of cellular homeostasis, regulating individual cellular processes and intercellular relationships. When cells do not interact properly or incorrectly decode molecular messages, a pathological process is triggered.
The main objective of our group is to understand the functional impact, at the cellular and organismal level, of new interactions of important G protein-coupled receptor (GPCR) signaling nodes, relevant in the maintenance of cellular homeostasis (eg. Gαq and GRK2), and how changes in them can affect the progression of pathologies, using cell and animal models with altered expression/activity of these proteins, as well as patient samples or animal models of disease. We will focus particularly on the functional impact of these new interactions and their modulation by accessory proteins (such as GRKs, AGS, RGS, caveolin, Ric8), on cell death processes, integration of nutrient sensing/autophagy signals, and endothelial dysfunction in the development of inflammatory/metabolic pathologies and cancer.
The Gαq interactome has expanded considerably with the description of new effectors and our group has contributed to this, through the identification of a new interaction region in Gαq, different from the classic effector binding region. This non-canonical Gαq signaling has turned out to be relevant in the development of cardiovascular pathologies and, furthermore, recent results have revealed the novel role of Gαq as a central modulator of mTORC1, contributing to the regulation of the autophagic process and thus to the maintenance of cell homeostasis, depending on nutrients fluctuations. These new Gαq interaction networks appear to be important in the regulation of endothelial function. Furthermore, Gαq is known to interact with various cytoskeletal components as well as important membrane microdomain organizers, suggesting the existence of signaling complexes that might be limited to specific subcellular environments.
In turn, we have also revealed a relevant role of one of the main regulators of these Gq-GPCR signaling pathways, GRK2, in the maintenance of the epithelial phenotype of stratified epithelia and demonstrated how its absence contributes to the development and malignancy of oral carcinomas (HNSCC), also revealing an important role of this kinase in epidermal homeostasis and in its inflammatory response.
To deepen a better understanding of the contribution of these signaling nodes to cellular communication between different cell types, both under physiological and pathological conditions, regulating exosome trafficking/autophagy, endothelial dysfunction/angiogenesis and extracellular matrix remodeling (including: normal or activated fibroblasts (CAF), endothelial cells, immune system cells and/or tumor cells), taking into account their respective secretomes, will contribute to the development of more effective therapies in inflammatory/metabolic and tumor contexts.
Some of these research objectives imply active collaborations with other members of our Unit at the CBMSO, as well as through CIBER Cardiovascular (CIBER-CV, ISCIII), Network of Biomedicine Integramune, (Comunidad de Madrid), as well as our affiliation to the Institute of Sanitary Research La Princesa.
Group members

Catalina Ribas Núñez
Lab.: 304 Ext.: 4728
cribas(at)cbm.csic.es

Inmaculada Navarro Lérida
Lab.: 304 Ext.: 4656
ilerida(at)cbm.csic.es

Ana Romo Gallo
Lab.: 320 Ext.: 4626
ana.romo(at)cbm.csic.es

Raquel Huertas Lárez
Lab.: 304 Ext.: 4656
raquel.huertas(at)cbm.csic.es

Virginia Ávila Oca
Lab.: 304 Ext.: 4656
vavila(at)cbm.csic.es

Natalia Maseda Bustillo
Lab.: 304 Ext.: 4656

Lizbeth Guadalupe Chávez Ramos
Lab.: Ext.: 4728
Selected publications
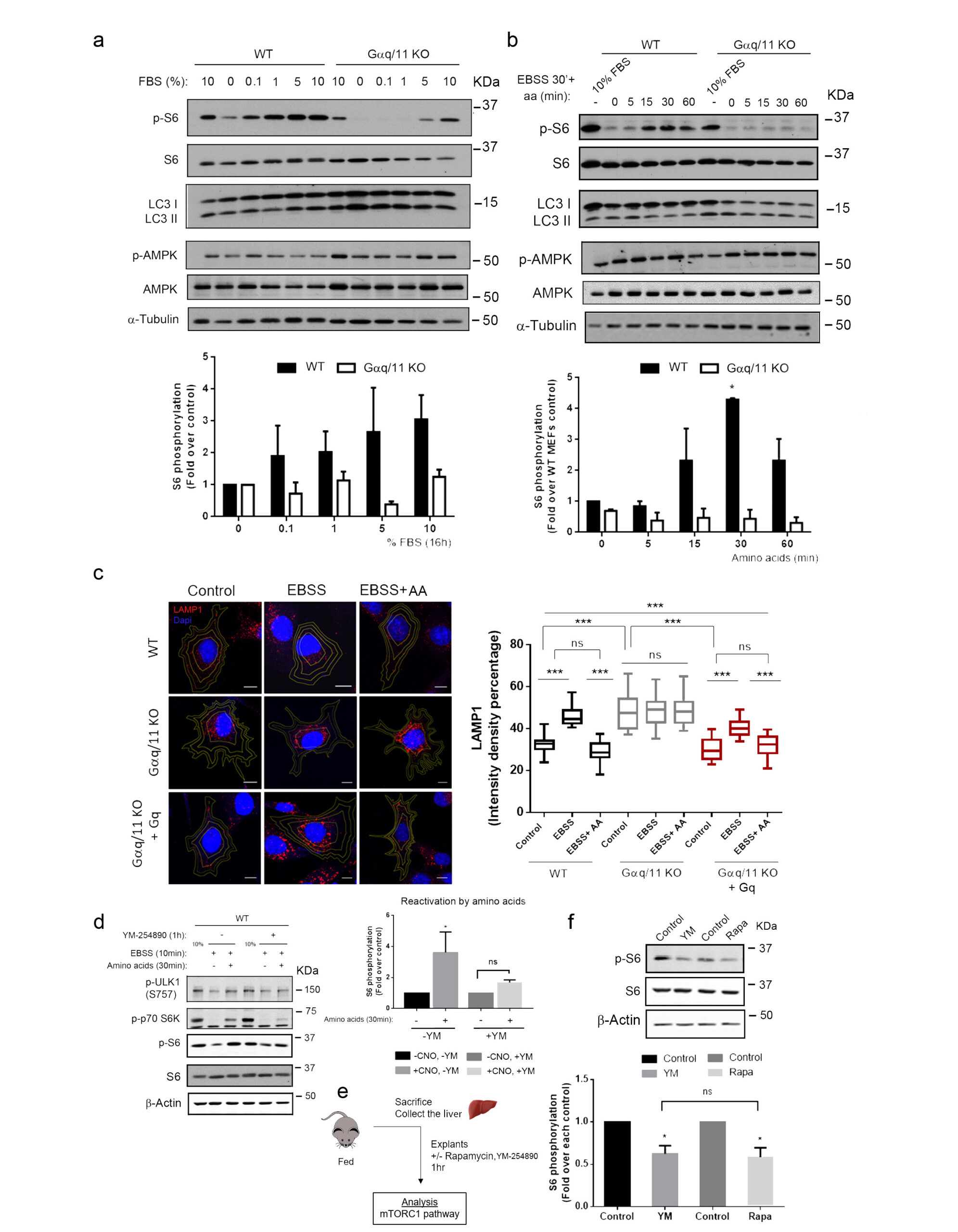
Gαq activation modulates autophagy by promoting mTORC1 signaling
Sofía Cabezudo et al.
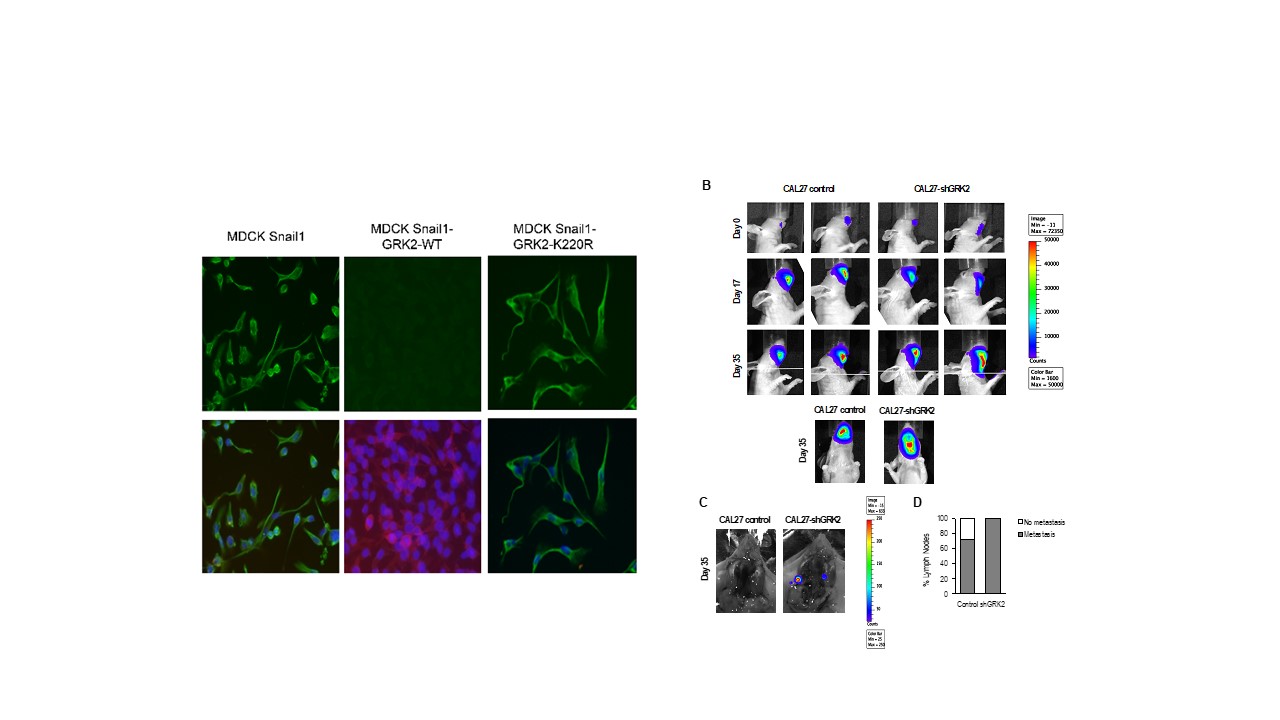
G-protein-coupled receptor kinase 2 safeguards epithelial phenotype in head and neck squamous cell carcinomas
Julia Palacios-García et al.
Gq Signaling in Autophagy Control: Between Chemical and Mechanical Cues
Inmaculada Navarro-Lérida et al.
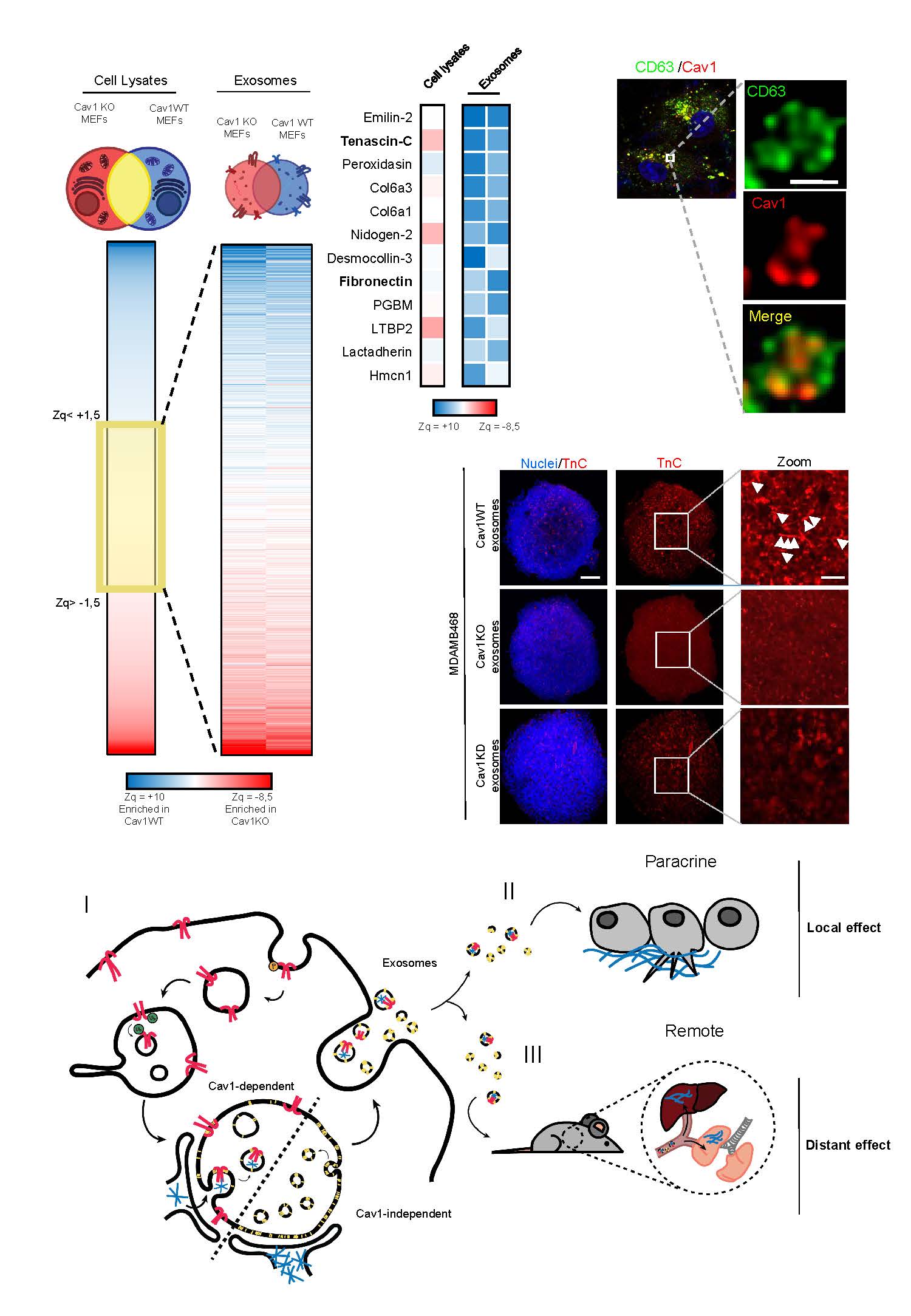
ECM deposition is driven by caveolin-1-dependent regulation of exosomal biogenesis and cargo sorting
Lucas Albacete-Albacete et al.





