Scientific Program
Physiological and pathological processes
RESEARCH GROUP
Cellular signaling networks in cancer (onco-resecel)

Petronila Penela Márquez
Cancer causes mortality due to metastatic progression and therapy resistance. Our focus is to understand how stress and obesity influence these processes, focusing on changes in the cell cycle, cellular identity, angiogenesis, and permissive microenvironments, through the post-translational rewiring of signaling by kinases and ligases in mammary gland, breast tumor cells, and endothelial cells

Research
Breast cancer is a disease of high prevalence with around 25,000 new diagnoses in Spain each year. Its incidence is much higher in the developed world, suggesting that the western lifestyle with unhealthy dietary habits (excessive caloric intake, overweight, obesity) and chronic stress states (adrenergic overstimulation) may influence the onset and progression of this disease.
The histopathological variety and molecular heterogeneity of breast tumors (luminal subtypes, triple negative, ERBB2), together with genomic instability and intra-tumoral cellular diversity, are factors that make difficult to achieve efficient treatments. In breast cancer, the main altered proteins responsible for genomic instability and heterogeneity are ATM kinase, Brca1 ligase and the tumor suppressor transcription factor p53 together with its negative regulator Mdm2 ligase. Although in ~ 80% of ductal breast carcinomas p53 is restrained by the activation/amplification of Mdm2 or by the deactivation of positive regulators such as ATM, therapies based on these targets are not yet satisfactory.
However, the identification of molecular dependencies in certain types of breast cancer has improved the treatment of patients with ERBB2(Her2) + or luminal tumors by using drugs directed against the HER2 tyrosine kinase receptor or the estrogen receptor (ER). However, in “triple negative” tumors (TNBCs) (negative for the steroid receptor (ER), progesterone (PR) and ERBB2 receptor) there are no clearly identified dependencies, and the treatments are not sufficiently effective. Another important problem is the emergence of resistance in luminal, Her2 and triple negative breast cancers, in parallel to the heterogeneity due to genomic instability and tumor metabolic reprogramming.
The objective of our group is to identify, as potential multifunctional therapeutic targets, signaling nodes that cooperate with oncogenes in the acquisition of tumor capacities (angiogenesis, proliferation, invasion or metastasis) or that block the activity of tumor suppressors, being able to alter the cellular homeostasis.
Results of our laboratory suggest that intertwined alterations of the serine-threonine kinase GRK2 and the Mdm2 ligase are key for cell-autonomous malignant transformation, as well as in the interplay of the transformed cell with the tumor micro-environment and the systemic condition of the patient. Our results indicate that these proteins modulate each other differently in normal epithelial cells and in tumor cells, responding in different ways to signals that stimulate adrenergic receptors and other G-protein coupled receptors (GPCR) or growth factor tyrosine kinase receptors (RTK).
In this context, our research aims to characterize the role of these nodes (GRK2 and Mdm2), as the relevant proteins phosphorylated and ubiquitinated by them, in a) diverse cellular processes such as cell cycle control and cell division, differentiation, energy metabolism or senescence, which are key in maintaining a normal cell behavior; b) in the consequences of hormonal (adrenergic, estrogenic) and metabolic stress on genomic stability; and c) in pro-tumoral stroma remodeling by analyzing the pathological angiogenesis and fibrosis that facilitate tumor growth and dissemination.
Group members

Laura Nogués Vera
Lab.: 320 Ext.: 4640
lnogues(at)cbm.csic.es

Petronila Penela Márquez
Lab.: 320 Ext.: 4640
ppenela(at)cbm.csic.es

Teresa González Muñoz
Lab.: 320 Ext.: 4652
teresa.gonzalez(at)cbm.csic.es

Clara Moyano Jimeno
Lab.: 320 Ext.: 4652
cmoyano(at)cbm.csic.es

Yazi Xu
Lab.: 320 Ext.: 4652

Ana Isabel Espinosa López
Lab.: 320 Ext.: 4640
Selected publications
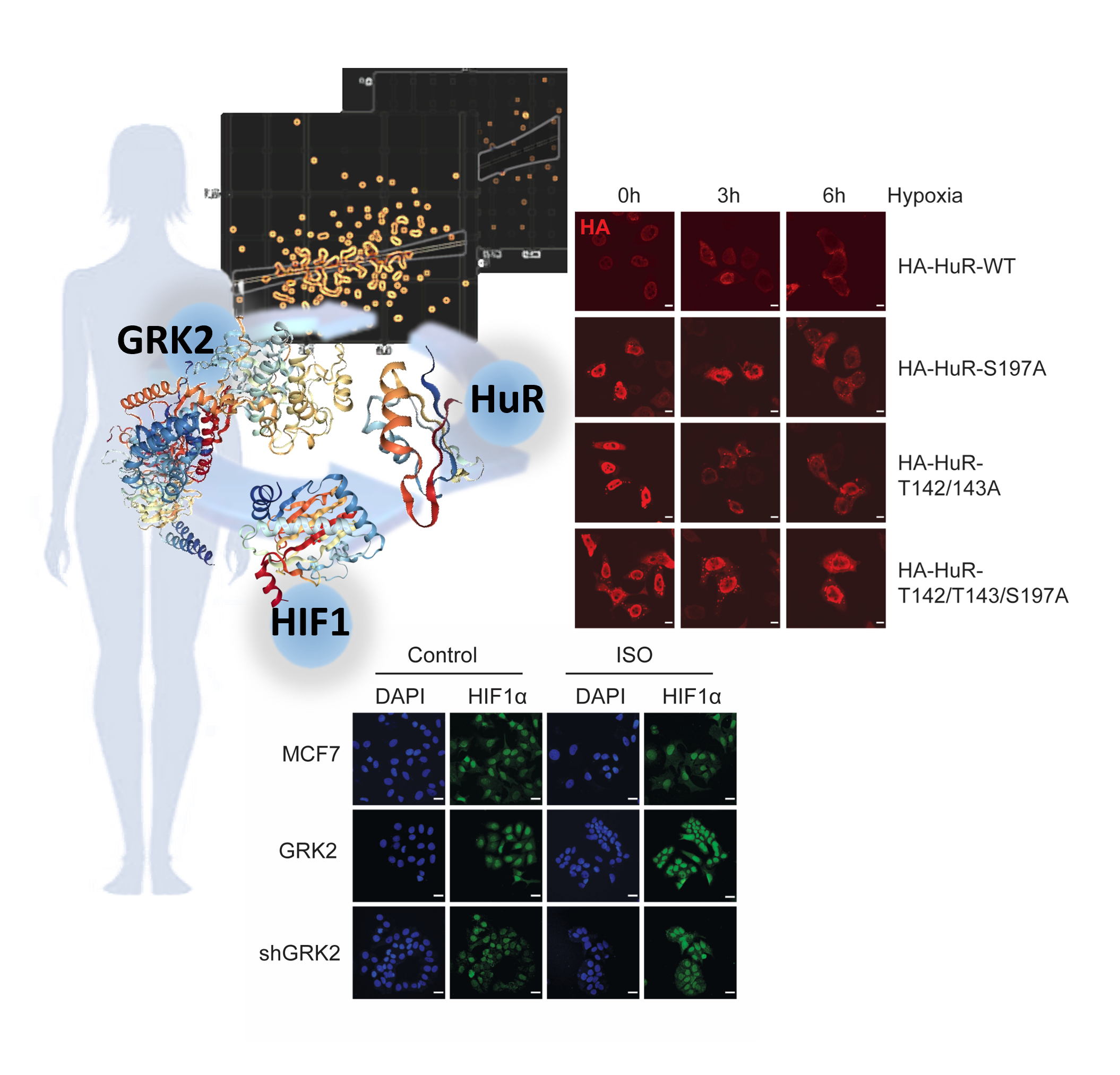
GRK2-Dependent HuR Phosphorylation Regulates HIF1α Activation under Hypoxia or Adrenergic Stress
Clara Reglero et al.
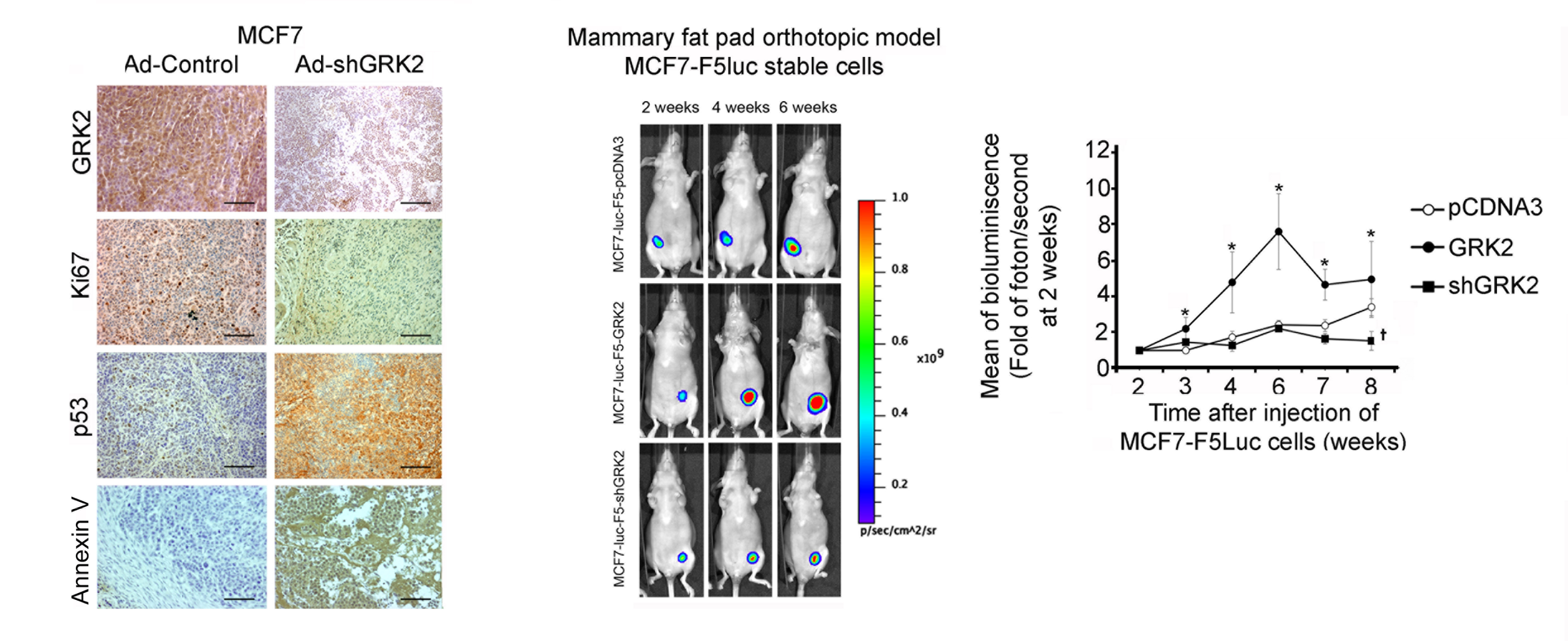
G Protein-coupled Receptor Kinase 2 (GRK2) Promotes Breast Tumorigenesis Through a HDAC6-Pin1 Axis
Laura Nogués et al.
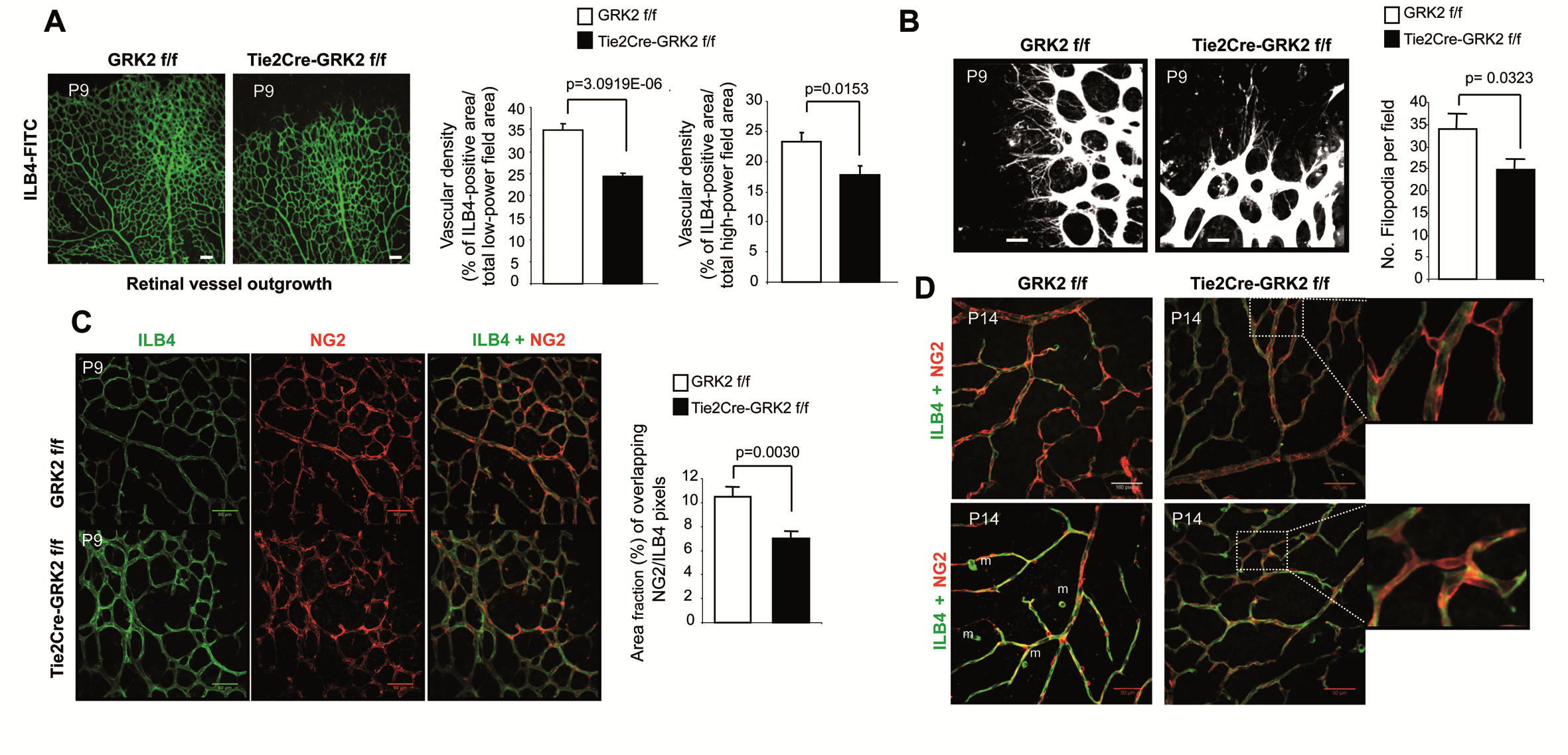
Developmental and tumoral vascularization is regulated by G protein–coupled receptor kinase 2
Verónica Rivas et al.
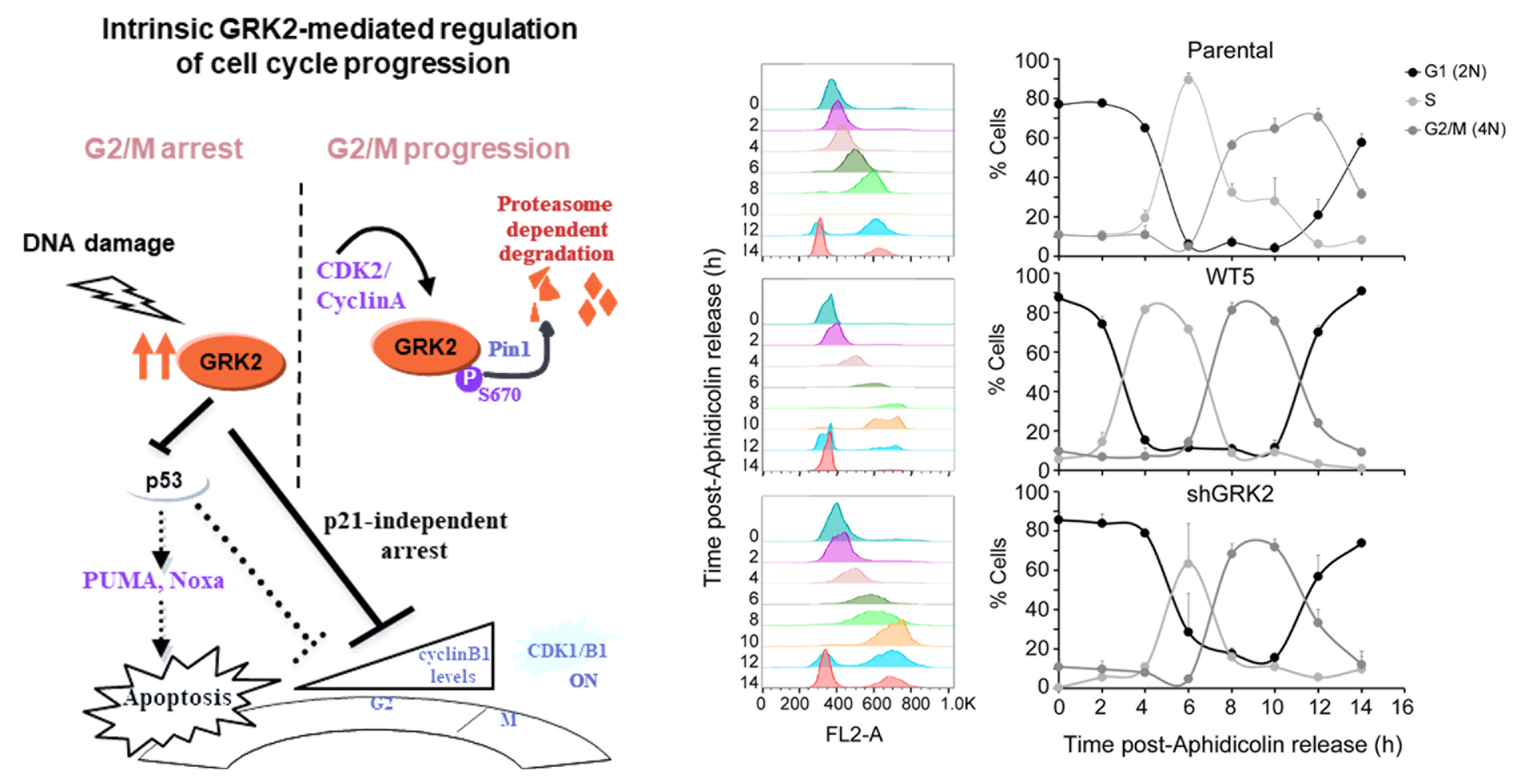
G protein–coupled receptor kinase 2 (GRK2) modulation and cell cycle progression
Petronila Penela et al.





