Scientific Program
Physiological and pathological processes
RESEARCH GROUP
Molecular pathophysiology of fibrosis

Santiago Lamas Peláez
The goal of our lab is to understand the molecular pathways leading to fibrogenesis, the accumulation of extracellular matrix components, mainly collagens, that occur in many human diseases. To this end, we have developed cellular models and biochemical approaches, as well as animal models, to explore the mechanisms underlying the metabolic changes associated to the development of kidney fibrosis.
Research
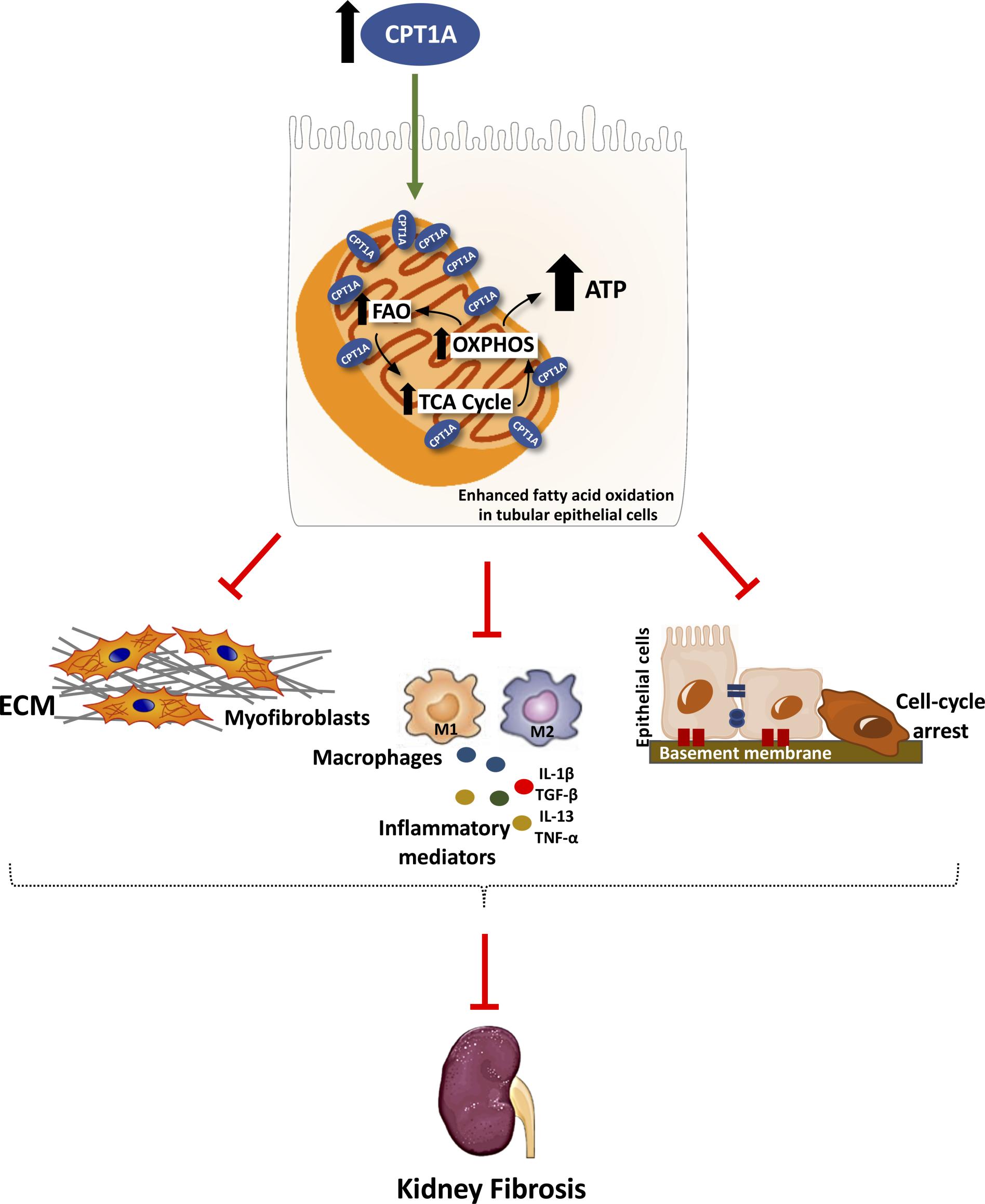
Organ fibrosis is a final common outcome for many diseases such as diabetic nephropathy, liver cirrhosis, scleroderma or myocardial sclerosis. It involves the replacement of cellular living tissue by extracellular matrix, with the subsequent functional derangement. Thus, it is crucial to understand the underlying molecular pathways leading to fibrogenesis in human pathology. MicroRNAs are essential post-transcriptional regulators of gene expression for multiple physiological and pathophysiological pathways, including fibrosis. During the past years we have focused on renal fibrosis regarding two questions: a) the role of metabolism in the genesis of renal injury and repair and b) the role of miRNAs involved in specific metabolic pathways critical for tubular epithelial cell function.
We aim to prevent, defer or revert fibrosis in the kidney by understanding underlying metabolic changes associated to kidney fibrosis. To this end we use animal models with specific gain-of-function for critical enzymes involved in fatty acid oxidation, such as carnitine palmitoyltransferase 1A (CPT1A). These studies are complemented by cellular models and biochemical approaches directed towards the study of mitochondrial biogenesis and function. We have found that overexpression of the enzyme Cpt1a in kidney tubules promotes enhanced fatty acid oxidation, restores mitochondrial homeostasis and protects from fibrosis. In addition, we have identified specific microRNAs that are important to regulate the genesis of fibrosis by targeting specific metabolic routes.
Group members

Santiago Lamas Peláez
Lab.: 103 Ext.: 4455
slamas(at)cbm.csic.es

Jessica Paola Tituaña Fajardo
Lab.: 103 Ext.: 4456
jtituana(at)cbm.csic.es

Belén Sirera Conca
Lab.: 103 Ext.: 4456
bsirera(at)cbm.csic.es
Selected publications
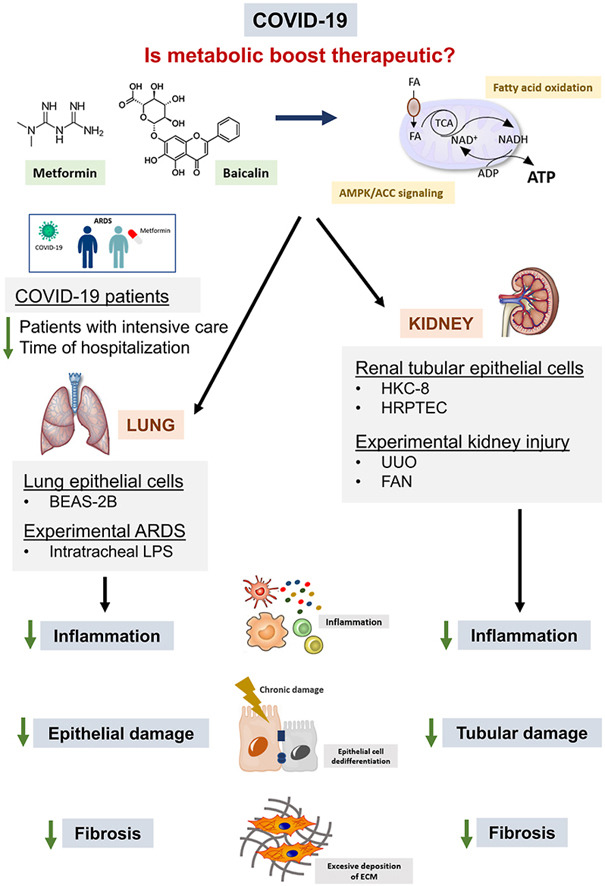
Enhanced fatty acid oxidation through metformin and baicalin as therapy for COVID-19 and associated inflammatory states in lung and kidney
Verónica Miguel et al.
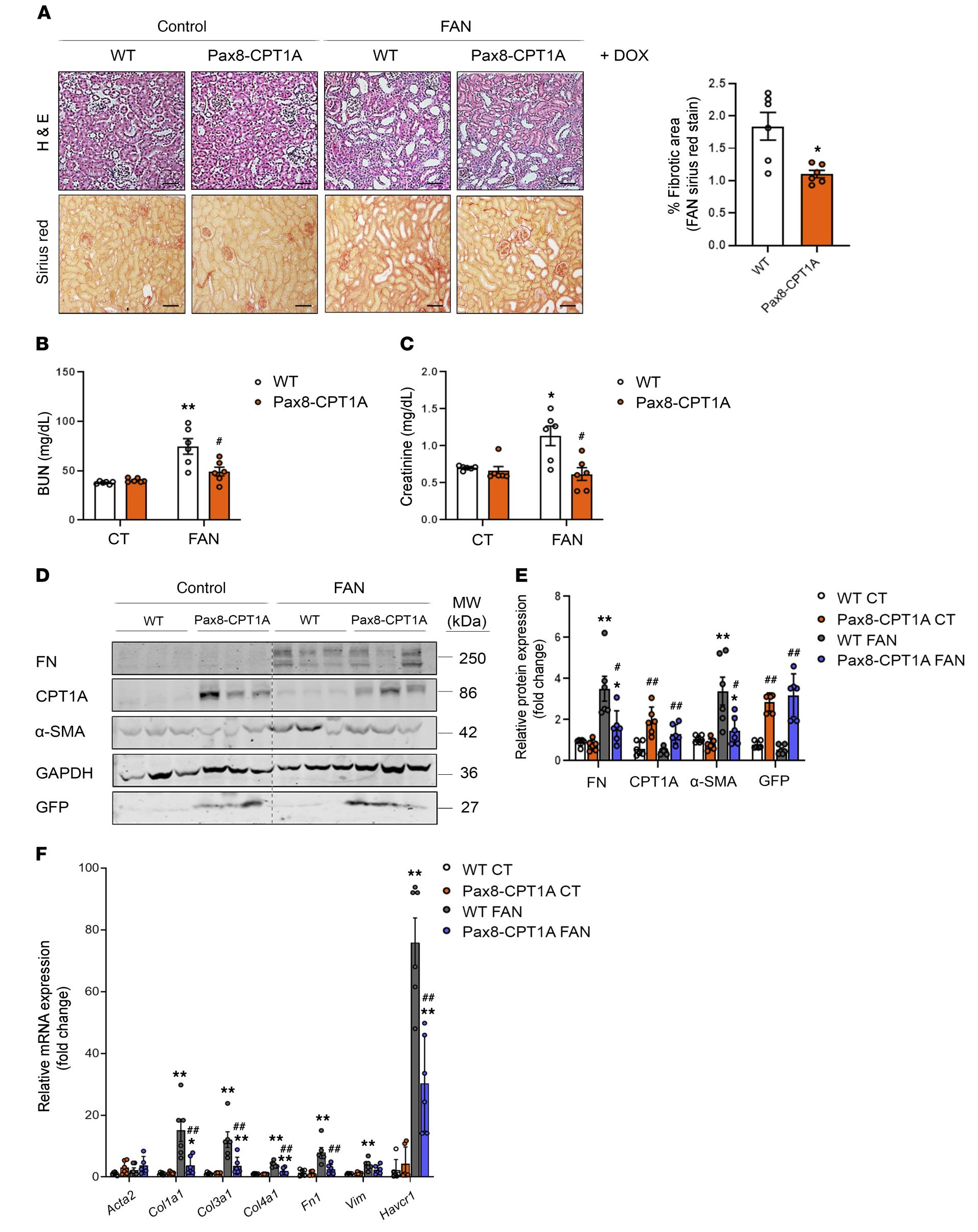
Renal tubule Cpt1a overexpression protects from kidney fibrosis by restoring mitochondrial homeostasis
Verónica Miguel et al.
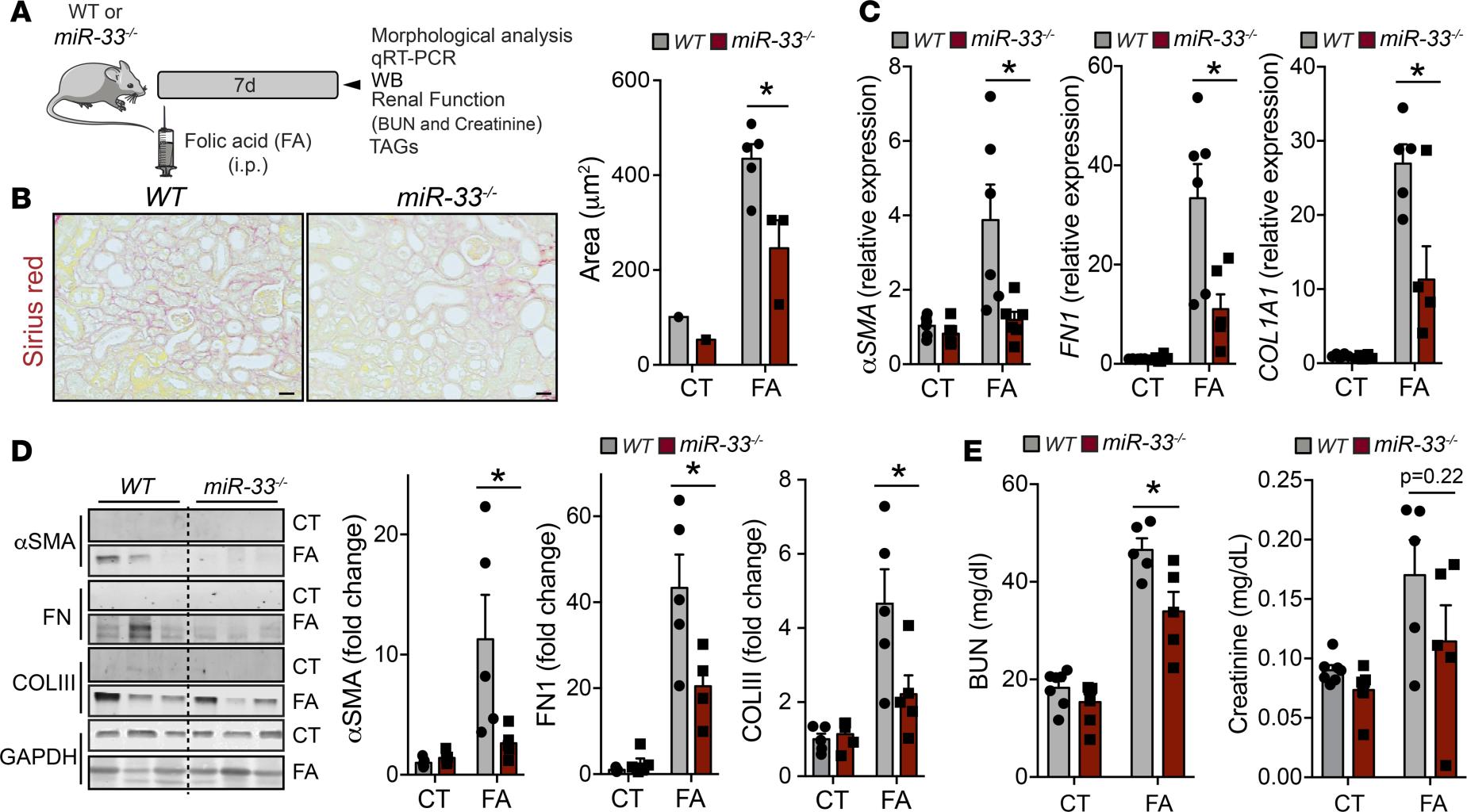
Genetic deficiency or pharmacological inhibition of miR-33 protects from kidney fibrosis
Nathan L. Price et al.
Reciprocal regulation between the molecular clock and kidney injury
Carlos Rey-Serra et al.





