Scientific Program
Tissue and organ homeostasis
RESEARCH GROUP
Morphogenesis and neurodegeneration of the vertebrate Central Nervous System

Paola Bovolenta
Why do some defects arise during the development of our brain, and why does our brain age or degenerate? Answering these key questions drives our research. These answers are crucial for eventually preventing or treating the numerous diseases that affect the nervous system, thereby avoiding their serious socio-economic consequence. We address these questions by combining different experimental models (zebrafish, mouse, and human organoids) with cellular and molecular biology approaches.

Research
The brain and other parts of the central nervous system (CNS), such as the retina, develop over a long period of time before reaching their final organization. Such a long period enables millions of neural cells to progressively acquire their specific identity and to progressively establish a precise network of functional connections among the generated cell types. Reaching such an elaborated organization is fundamental for our daily life and well-being. As we age, the brain again undergoes progressive changes but unfortunately “in the reverse direction”, making us less proficient. When these changes are drastic, as it happens in people suffering from Alzheimer’ disease or in individuals who becomes blind because their “photoreceptors” degenerate, performing even simple duties or relating to the immediate surroundings become impossible. Knowing how to have and preserve a healthy brain thus falls in between the comprehension of its development and the identification of the causes of its ageing. Our laboratory divides its research efforts between these two extremes. On one side we investigate a) how the visual system forms; on the other, b) we search the molecular causes underlying the brain alterations occurring in aging and/or Alzheimer ‘disease.
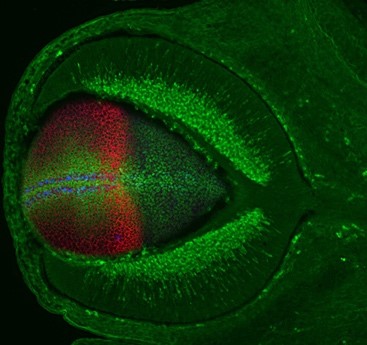
a) For over twenty years, our team has contributed to identify and study the molecular building blocks that coordinate the development of the vertebrate visual system. Building on this experience, we are now investigating how the retinal pigmented epithelium (RPE) forms and contributes to the overall architecture of the eye. The RPE consists of a monolayer of cells, positioned at the back of the neural retina. This tissue has the critical function of protecting, nourishing and cooperating with the light sensing cells of the retina: the photoreceptors. The cells of the RPE are initially neuroepithelial as those of the neural retina but soon transform in a cuboidal/squamous pigmented layer (Figure 1). The events underlying this transformation are still poorly understood. Using the zebrafish and chick embryos as models, we are studying the cellular and molecular aspects of this transformation and are further asking whether this transformation is necessary for the acquisition of the final cup-shape that the vertebrate eye has. In other words, we want to know what happens to the eye if the RPE does not develop correctly. As an extension of this question, we are also asking how the entire visual system adapts to the abnormal development of one of its parts. For example, what happens to the eye if the thalamic visual structures do not form in an appropriate manner?
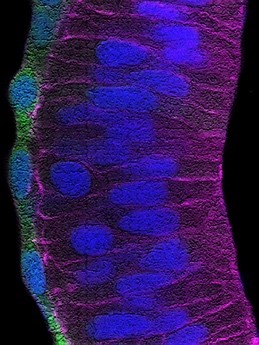
b) When the brain ages, the number of functional connections among neurons decreases and the brain assume a state of low but chronic “inflammation”. These changes are exacerbated in neurodegenerative diseases such as Alzheimer’s Disease (AD), in which there is also an accumulation of typical “amyloid plaques” (Figure 2). From a molecular point of view, loss of connections, inflammation and amyloid plaque formation have a commonality: the activity of the metalloprotease ADAM10. We have recently shown that a small secreted protein, known as Secreted Frizzled Related Protein 1 (SFRP1), acts as an endogenous inhibitor of ADAM10. Based on this observation, we hypothesized that abnormally upregulated SFRP1 levels in the brain of AD patients could impair ADAM10 activity and be a common trait of AD. Our work has validated this hypothesis, demonstrating that SFRP1 contributes to AD pathogenesis, whereas neutralization of its activity in a mouse model prevents the progression of the disease and the loss of memory present in AD (or in aging). Based on this exciting result and taking advantage of different transgenic mouse models that we have generated, we are now investigating the direct implication of Sfrp1 in the loss of brain connections and in the inflammatory events that take place in AD and aging. We are further exploring the potential of SFRP1 neutralization as a therapeutic strategy against AD.
Group members

Mª Jesús Martín Bermejo
Lab.: 426 Ext.: 4720
cbermejo(at)cbm.csic.es

Paula Bovolenta Nicolao
Lab.: 426 Ext.: 4718
pbovolenta(at)cbm.csic.es

Noemí Tabanera Anguita
Lab.: 426 Ext.: 4720
ntabanera(at)cbm.csic.es

María Elena Sánchez Bustamante
Lab.: 426 Ext.: 4720
elena.sanchez(at)externos.cbm.csic.es

Pablo Miaja Hernández
Lab.: 426 Ext.: 4720
pablo.miaja(at)cbm.csic.es

Marcos Martínez Baños
Lab.: 426 Ext.: 4720
mmartinez(at)cbm.csic.es

Eva Pajda Szeligowska
Lab.: 426 Ext.: 4720
eva.pajda(at)cbm.csic.es

Adrián Pérez Ramos
Lab.: 426 Ext.: 4720
adrian.perez(at)cbm.csic.es

Eleonora D´Ambra
Lab.: 426 Ext.: 4720
edambra(at)cbm.csic.es

Aikaterini Karkali
Lab.: 414 Ext.: 4465
k.karkali(at)cbm.csic.es
Selected publications
SFRP1 modulates astrocyte‐to‐microglia crosstalk in acute and chronic neuroinflammation
Javier Rueda‐Carrasco et al.

Cdon acts as a Hedgehog decoy receptor during proximal-distal patterning of the optic vesicle
Marcos Julián Cardozo et al.

Elevated levels of Secreted-Frizzled-Related-Protein 1 contribute to Alzheimer’s disease pathogenesis
Pilar Esteve et al.
Stretching of the retinal pigment epithelium contributes to zebrafish optic cup morphogenesis
Tania Moreno-Mármol et al.





