Scientific Program
Tissue and organ homeostasis
RESEARCH GROUP
Transcriptional control of sexual differentiation of the nervous system

Esther Serrano-Saiz
Sex differences are a defining feature of many neuropsychiatric disorders, influencing onset, prevalence, symptoms, and treatment outcomes. The biological underpinnings of these differences remain largely unresolved. Sexual differentiation of the nervous system depends on the precise temporal and spatial regulation of gene expression, which is coordinated by sex hormones and genetic factors at various developmental stages and anatomical sites. Disruptions in these processes can amplify vulnerability to neuropsychiatric conditions. Unraveling the genetic and molecular pathways that shape sex-dependent neuronal identity, connectivity, and plasticity is crucial for understanding their links to neuropsychiatric diseases.
Research
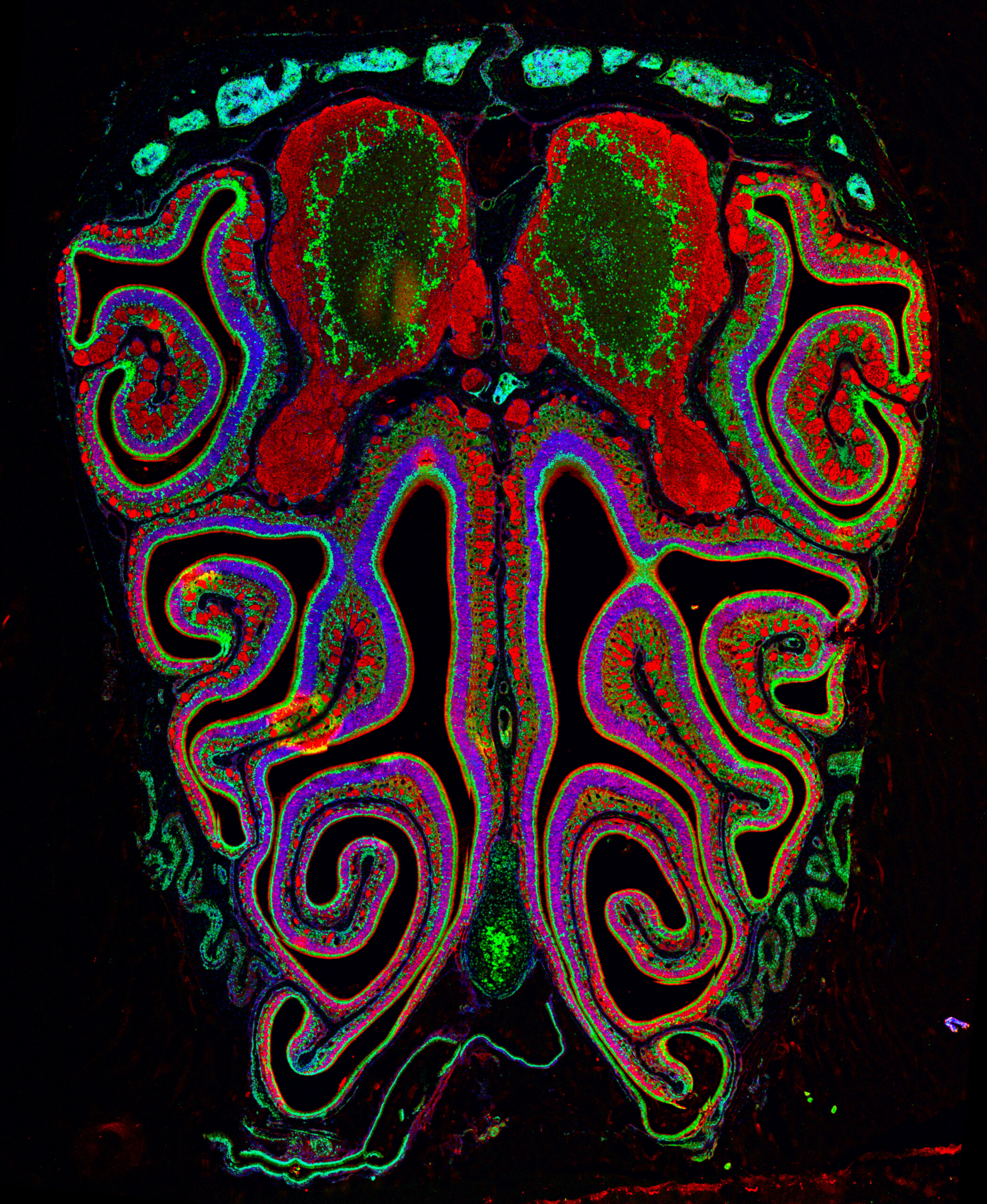
The sexual differentiation of the mammalian brain has been primarily attributed to the effects of sex hormones. However, a growing body of evidence demonstrates that genetic and epigenetic factors also play key roles in shaping sexual differences. DMRT transcription factors are conserved master regulators of sex-specific traits across animal species. However, their functions in the mammalian nervous system are not yet fully understood. Our research explores how DMRTs govern sex-specific neuronal identities, cell numbers, circuit connectivity, and overall transcriptional regulation in the context of sex-linked genetic mechanisms.
Over the past few years, we have built a comprehensive expression atlas for all Dmrt genes in the mouse brain, comparing sexes and across development (MGI repository). We provide the first description of neuronal expression for Dmrt2 and Dmrt6, and identify Dmrt5 in postnatal neurogenic niches. While mammalian Dmrts do not display sexually dimorphic expression, notable quantitative differences in expression levels between sexes emerged. Moreover, most Dmrt genes were maintained in postmitotic neurons. Together, these findings open up novel avenues for the unrecognized roles of Dmrt factors in postnatal neurogenesis and brain sexual differentiation.
We are approaching these questions using genetic models, transcriptomic approaches, and advanced microscopy techniques in both mouse models and human organoids. Our work will unravel novel principles of brain sexual differentiation. It will generate mouse models to better understand genetic mechanisms that either afford protection or generate vulnerability in the etiology and sexual bias of mental disorders.
Group members

Esther Serrano Saiz
Lab.: 425 Ext.: 4732
eserrano(at)cbm.csic.es

Rafael Casado Navarro
Lab.: 425 Ext.: 4464
rcasado(at)cbm.csic.es

Rodrigo Torrillas de la Cal
Lab.: 425 Ext.: 4464
rtorrillas(at)cbm.csic.es

Miguel Rubio García
Lab.: 425 Ext.: 4464
mrubio(at)cbm.csic.es

Andrea Arroyo Almeda
Lab.: 425 Ext.: 4464
aarroyo(at)cbm.csic.es

Carla Aurora Domínguez Martínez
Lab.: 425 Ext.: 4732
Selected publications

Dmrt2 regulates sex-biased neuronal development in the cingulate cortex
Bermejo-Santos A et al.

DMRT Transcription Factors in the Control of Nervous System Sexual Differentiation
Casado-Navarro R et al.

Sexually Dimorphic Differentiation of a C. elegans Hub Neuron Is Cell Autonomously Controlled by a Conserved Transcription Factor
Esther Serrano-Saiz et al.

Single-cell molecular and developmental perspectives of sexually dimorphic circuits underlying innate social behaviors
Esther Serrano-Saiz and Isogai Y
Latest publications
Former lab members
- Ana Bermejo-Santos (PhD 2025)
- Ana J. Candel (TFM 2025)
- Miguel Rublio (TFM 2024)
- Virgilia Olivé (TFM 2024)
- Emilio Martina Perez (TFM 2023)
- Julia Berges (TFM 2022)
- Denisa Lupu (TFM 2020)
- Rafael Casado-Navarro (TFM 2020)






