Scientific Program
Tissue and organ homeostasis
JUNIOR PI
Chromosome instability, cancer and immunity

Carolina Villarroya Beltri
Research
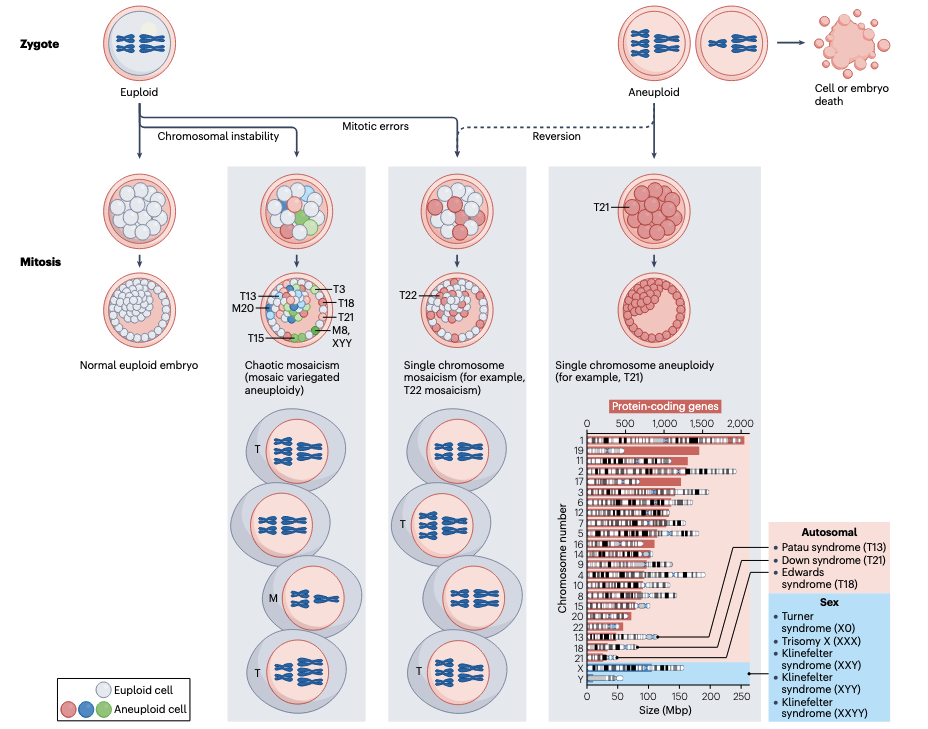
Chromosome Instability is the increased propensity to commit errors in the segregation of chromosomes during cell division. As a consequence, cells with chromosome instability frequently gain or lose chromosomes, generating aneuploid daughter cells. Aneuploidy, which is the condition of having an abnormal number of chromosomes, is found in approximately 90% of human tumors, while it is extremely rare in normal tissues. Multiple lines of evidence indicate that aneuploidy is not only a hallmark of cancer but also a driving force; however, aneuploidy has been historically treated as a passive consequence of cancer, and its potential as a therapeutic vulnerability remains largely unexplored. In our laboratory we aim to decipher how chromosomal instability and cellular stress responses shape tumor adaptation, therapeutic resistance and interactions with the immune system.
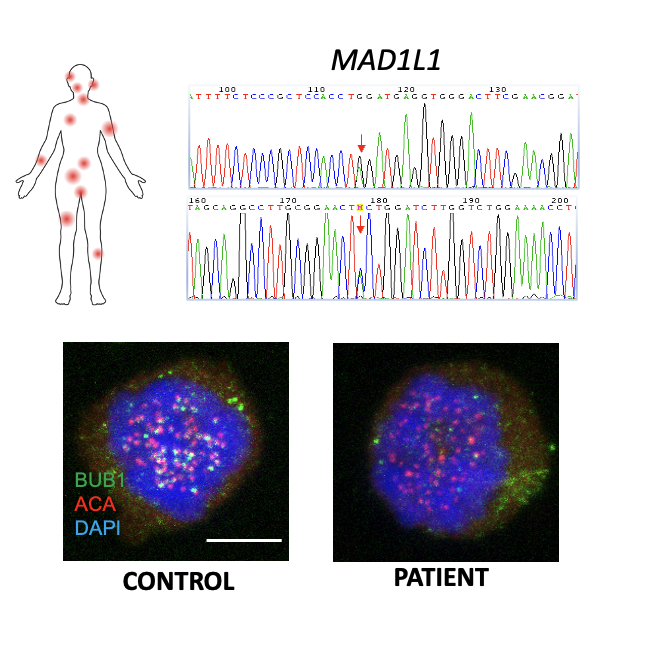
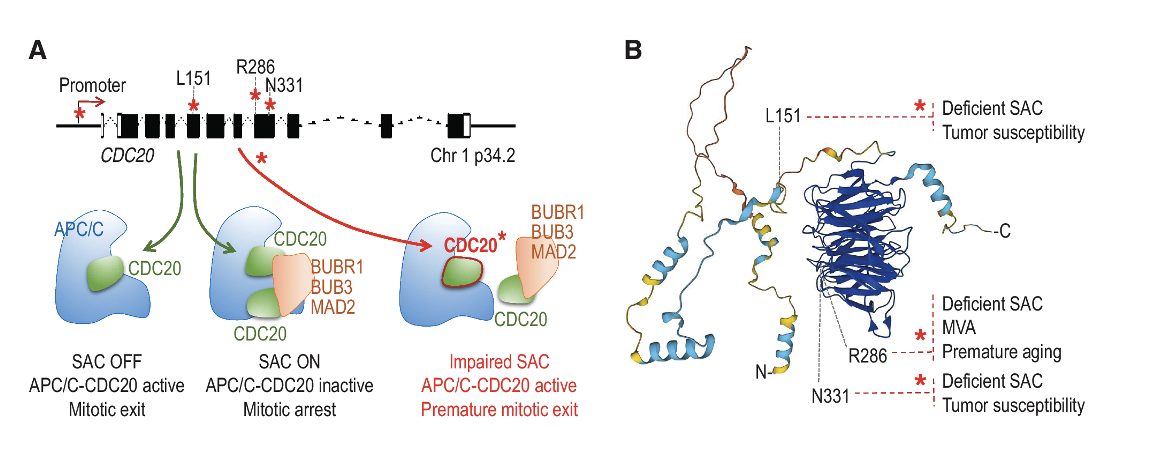
The most immediate molecular consequence of gaining or losing one copy of a chromosome is an imbalance in the expression of the genes encoded by the chromosome that is gained or lost. As a consequence, aneuploid cells exhibit gene expression changes and a stress response that usually result in reduced proliferation and IFN upregulation, irrespective of the affected chromosome. However, despite these initial fitness costs, aneuploid cells can eventually adapt, giving rise to clones with selective advantages. In our laboratory we want to understand how aneuploid cells adapt to this condition, and how this aneuploidy-stress response affects their interaction with the immune system. For that we work with cellular and mouse models of chromosome instability, and with human samples of patients with Mosaic Variegated Aneuploidy (MVA). These individuals have mutations in genes encoding proteins involved in the proper segregation of chromosomes during mitosis and, as consequence, aneuploid cells affecting different chromosomes are continuously generated and account for 10-40% of their total cells. Therefore, these patients represent a unique scenario to understand the consequences of aneuploidy in humans, including its role in cell transformation as well as the interaction of the aneuploid cells with the immune system. In addition, we apply CRISPR-based technologies to understand how cell stress responses shape tumor resistance to different targeted-therapies. By dissecting how these stress-adaptive programs are engaged during aneuploidy and in response to targeted therapies, our work aims to identify context-specific vulnerabilities that can be exploited for the development of novel, mechanism-based cancer therapies.
Selected publications
Mosaic variegated aneuploidy in development, ageing and cancer
Malumbres, M. et al.
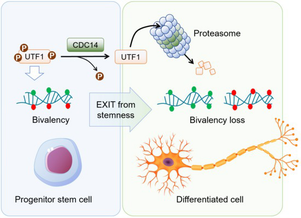
Mammalian CDC14 phosphatases control exit from stemness in pluripotent cells
Villarroya-Beltri, C. et al