Scientific Program
Tissue and organ homeostasis
RESEARCH GROUP
Molecular and translational research in vascular pathologies

Juan Miguel Redondo Moya
Our lab investigates the molecular mechanisms underlying vascular diseases, including hereditary conditions such as Marfan and Loeys-Dietz syndromes. We study signaling pathways and key mediators and conduct proteomic analyses to identify biomarkers and therapeutic targets, aiming to improve the diagnosis, prognosis, and treatment of these conditions.
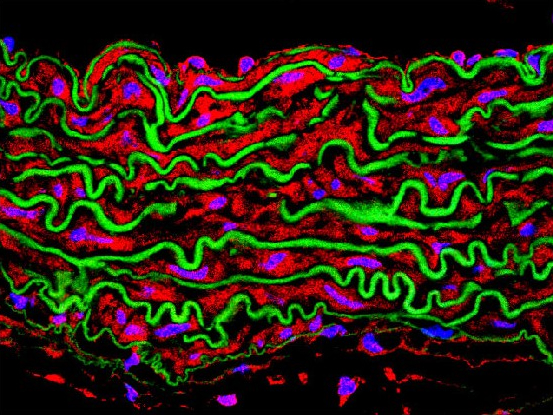
Research
In recent years, our research has focused on the molecular and cellular mechanisms driving vascular remodeling and aortic pathophysiology, particularly in heritable thoracic aortic diseases (HTAD).
Aortic diseases cause 1%-2% of deaths in industrialized countries. Aortic aneurysm (AA) leads to progressive dilation and medial degeneration, often culminating in acute dissection (AD), a life-threatening event. In HTAD such as Marfan syndrome (MFS) and Loeys-Dietz syndrome (LDS), aortic dilation increases AD risk, yet medical treatments and risk predictors remain very limited due to gaps in understanding their molecular mechanisms. Surgery is the only effective intervention, guided by diameter thresholds, but AD can still occur at smaller diameters. There is an urgent need to (i) deepen mechanistic understanding of HTAD, (ii) identify reliable risk markers beyond aortic diameter, and (iii) develop pharmacological strategies.
In collaboration with Miguel Campanero’s group, we identified key HTAD mediators, including Adamts1, Nos2, C/EBPβ, Plk1, and Rcan1. Adamts1 deficiency induces aortic disease via NOS2-mediated NO overproduction, and NOS2 inhibitors prevent and reverse pathology in MFS and Adamts1 mouse models. We also found that the NO-sGC-PKG pathway is overactivated in MFS due to extracellular matrix protein accumulation, including Versican (Vcan) and Fibronectin (FN), which activate the AKT-NOS2 pathway. Genetic Vcan deficiency, lentiviral silencing, or AKT inhibition reversed aortic dilation, highlighting new therapeutic targets. Additionally, FN accumulation promotes MFS aortopathy via integrin signaling, initiating a cascade we have characterized in detail. Mapping these pathways represents a major breakthrough, establishing the most comprehensive signaling network in MFS aortopathy to date and linking extracellular matrix alterations with AKT-NO and integrin-mediated signaling. Our data also suggest that the AKT-NOS2 pathway, previously linked to MFS, also plays a role in LDS, warranting further investigation.
Furthermore, in collaboration with Jesús Vázquez’s group at CNIC, we conducted proteomic analyses that identified sex-dependent and independent molecular signatures in MFS and LDS, revealing shared patterns absent in controls and distinct clusters differentiating the syndromes. This work has enabled us to identify common mediators, syndrome-specific proteins, and sex-associated markers, all of which require further characterization. Additionally, we identified plasma proteins in MFS patients with high diagnostic accuracy and correlation with clinical severity, supporting their validation as diagnostic and prognostic biomarkers.
Overall, our research aims to provide new insights into HTAD pathophysiology and open avenues for therapeutic target discovery and biomarker development in aortic diseases.
Group members

Juan Miguel Redondo Moya
Lab.: 311 Ext.: 4451
jmredondo(at)cbm.csic.es

María José Méndez Olivares
Lab.: 311 Ext.: 4447
mjmendez(at)cbm.csic.es

Sara Martínez Martínez
Lab.: 211 Ext.: 4401
smartinez(at)cbm.csic.es

Iván Alarcón Ruíz
Lab.: 311 Ext.: 4451
ivan.alarcon(at)cbm.csic.es
Selected publications
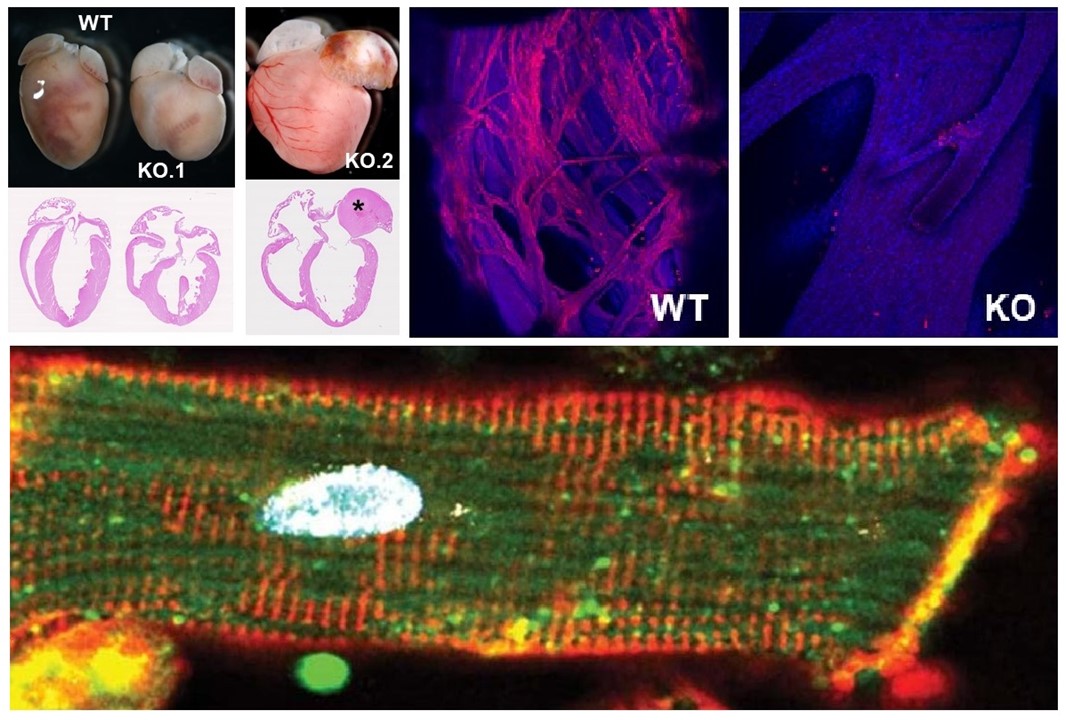
The G4 resolvase Dhx36 modulates cardiomyocyte differentiation and ventricular conduction system development
Gómez-Del Arco P et al.
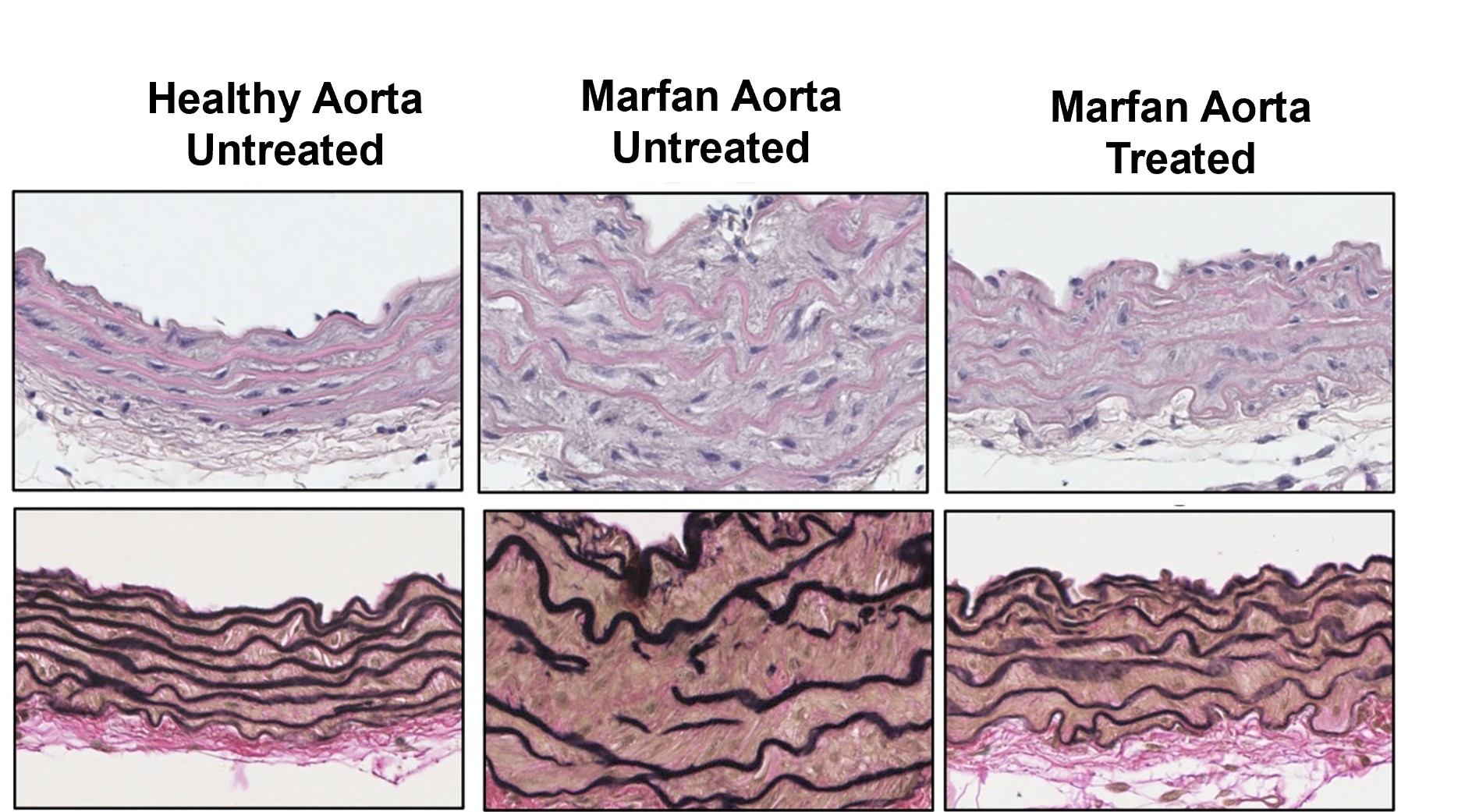
Aortic disease in Marfan syndrome is caused by overactivation of sGC-PRKG signaling by NO.
de la Fuente-Alonso A et al.
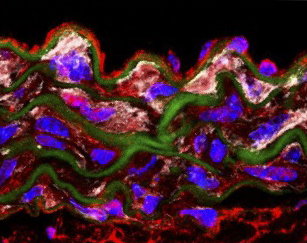
Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome.
Oller J et al.
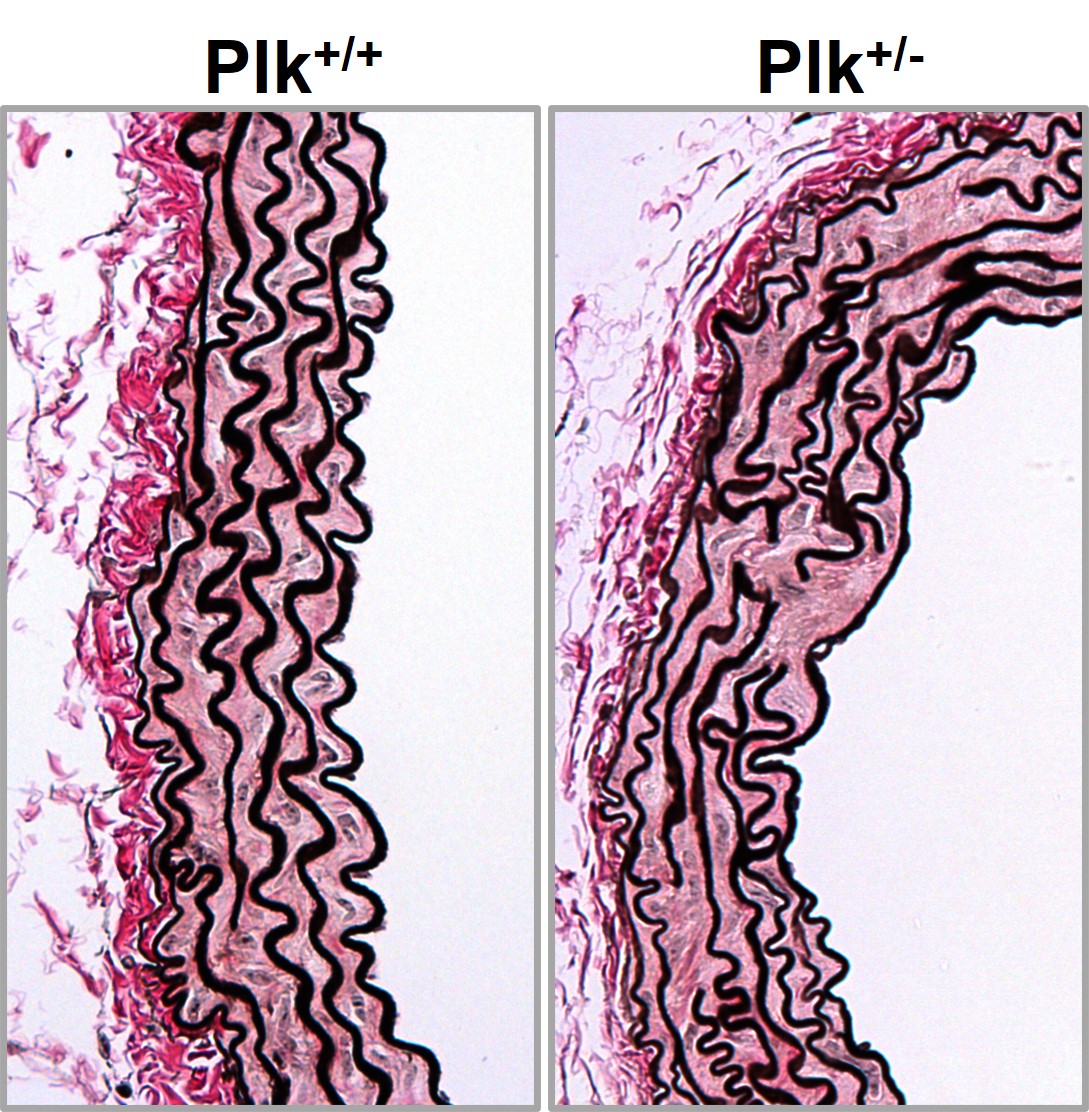
Plk1 regulates contraction of postmitotic smooth muscle cells and is required for vascular homeostasis.
de Cárcer G et al.





