Scientific Program
Tissue and organ homeostasis
RESEARCH GROUP
Pathophysiology of peritoneal inflammation and fibrosis (PERINFIB)

Manuel López Cabrera
Myofibroblasts and carcinoma-associated fibroblasts (CAFs) are considered major contributors for peritoneal fibroproliferative response and for niche preparation during peritoneal metastasis. Our group has demonstrated that the majority of peritoneal myofibroblasts and CAFs derive from the mesothelial cell through an epithelial-to-mesenchymal transition (EMT). Our aims during the last years has been the search of prognostic/diagnostic as well as therapeutic tools to improve the outcomes of diseases affecting the peritoneal membrane (peritoneal dialysis (PD)-induced fibrosis, postsurgical adhesions and peritoneal carcinomatosis) by identifying common mechanisms and interventions that promote long-term preservation of peritoneal membrane function and survival in life threatening conditions of the peritoneum.
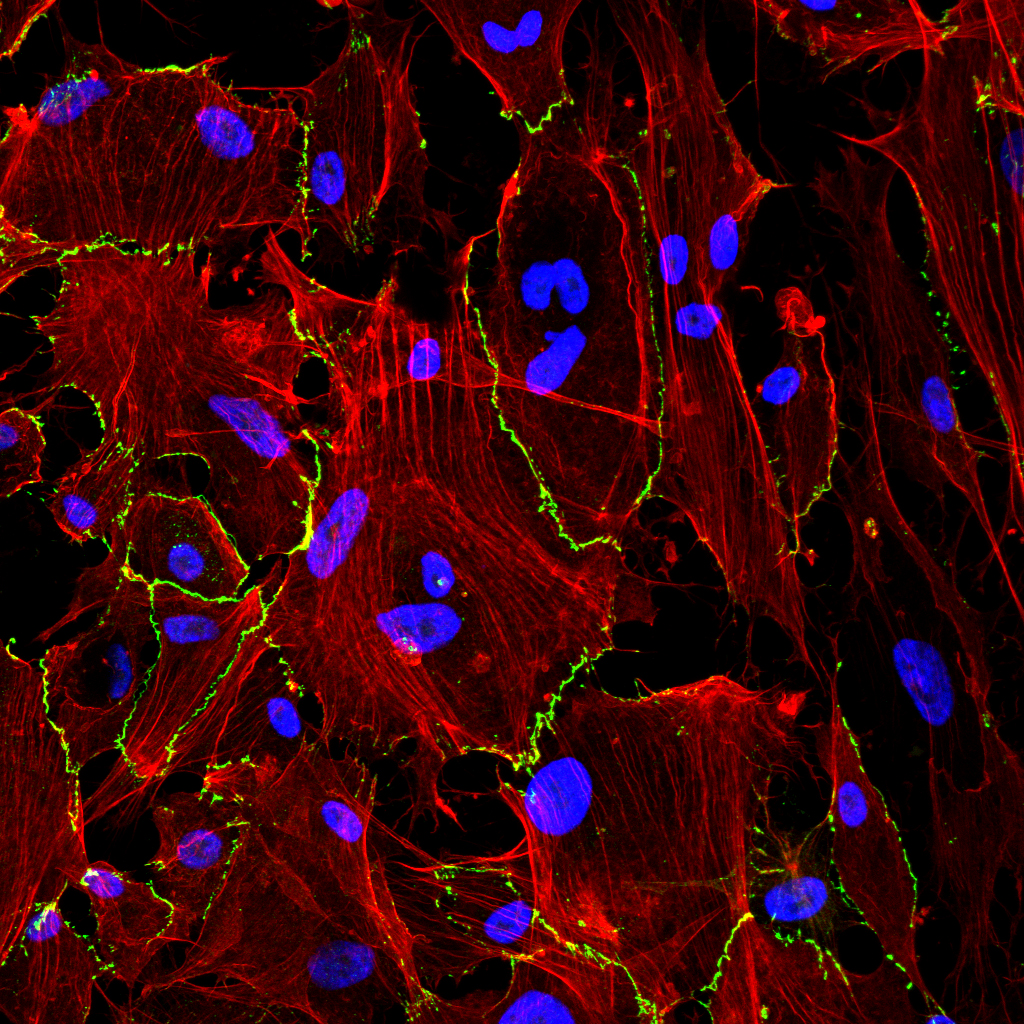
Research
Used to treat advanced chronic kidney disease, peritoneal dialysis (PD) consists of using the peritoneum as a semipermeable membrane across which diffusion and ultrafiltration take place. During PD, the peritoneum is exposed to bio-incompatible solutions that cause inflammation, angiogenesis and fibrosis, resulting in membrane failure. Our group has shown that mesothelial cells undergo an EMT in response to the peritoneal insult. During the last 16 years, there have been reports in the literature that mesothelial EMT (also termed mesothelial-to-mesenchymal transition, MMT) is a good marker for membrane failure and a therapeutic target for preventing PD-induced fibrosis and/or angiogenesis.
Recently, we considered whether the MMT process plays a role in other peritoneal pathologies such as peritoneal metastasis and postsurgical adhesions. Peritoneal metastasis is a complication of abdominal carcinomas (e.g. ovarian carcinoma) for which there is no effective therapy. Progression of the metastatic implants is affected by CAFs, which can derive from different cell types. We have shown in human peritoneal implant biopsies that a subpopulation of CAFs derives from mesothelial cells through MMT. Our results also suggest that MMT renders the peritoneum more receptive to implantation of tumor cells, contributes to the growth and vascularization of secondary tumors and that MMT is a therapeutic target in peritoneal carcinomatosis.
Adhesions are areas of fibrotic tissue that bind tissues and organs that would normally not be connected, and can be seriously life-threatening. Most adhesions are postsurgical. Histological analyses of human postsurgical adhesions have demonstrated that the mesothelial cells adjacent to the fibrotic tissue show signs of an MMT, suggesting that this could be an initial step in their development. The physiopathological processes involved in their formation remain unknown. Recent results have shown that mechanical injury is the main inducer of MMT during the formation of adhesions. Consequently, molecules involved in mechano-transduction, such as caveolin 1 (CAV-1), could play a role in the modulation of mechanical MMT and adhesion formation.
The aim of our work is to expand the knowledge of the pathological implications of the MMT of mesothelial cells and the molecular mechanisms that regulate this process, and to identify molecular targets for the design of therapeutic strategies, with possible applications in diseases associated with peritoneal fibrosis/angiogenesis, and in peritoneal metastasis.
Group members

Manuel López Cabrera
Lab.: 406 Ext.: 4604/4707
mlcabrera(at)cbm.csic.es

Mª Pilar Sandoval Correa
Lab.: 406 Ext.: 4707
psandoval(at)cbm.csic.es

Henar Tomero Sanz
Lab.: 406 Ext.: 4707
htomero(at)cbm.csic.es
Selected publications
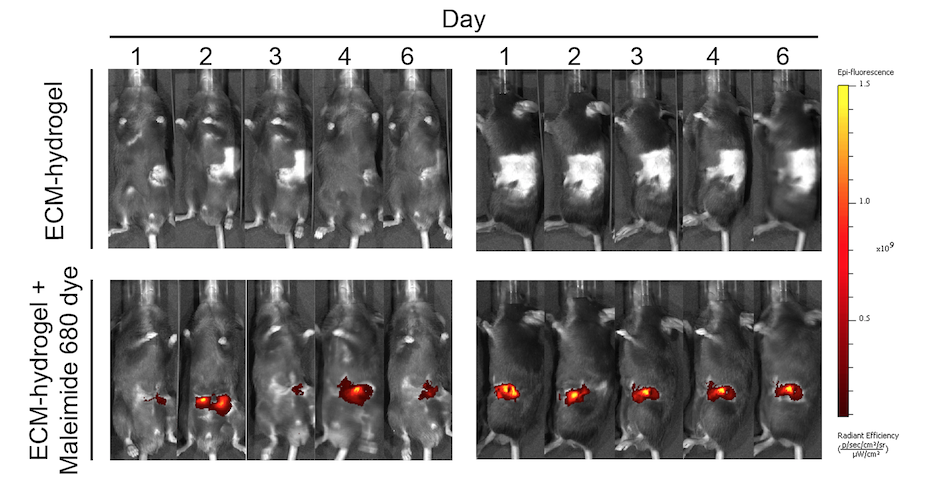
Sprayable extracellular matrix hydrogel reduces postoperative adhesion formation and protects healing tissues in preclinical models
Lucía Pascual-Antón, Pilar Sandoval et al. Science Translational Medicine, 2025.
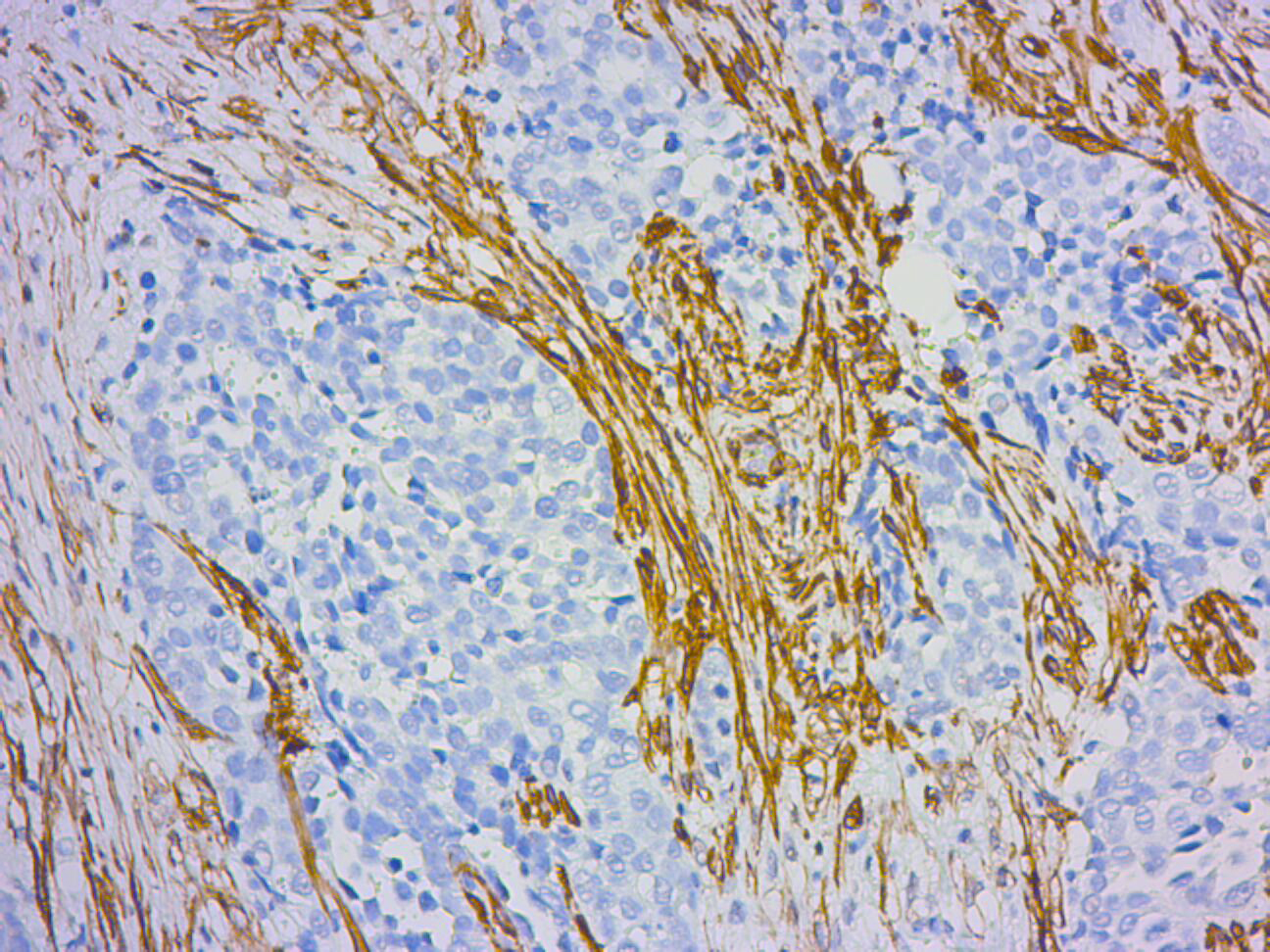
Targeting carcinoma-associated mesothelial cells with antibody-drug conjugates in ovarian carcinomatosis
Lucía Pascual-Antón, Pilar Sandoval et al. The Journal of Pathology, 2023.
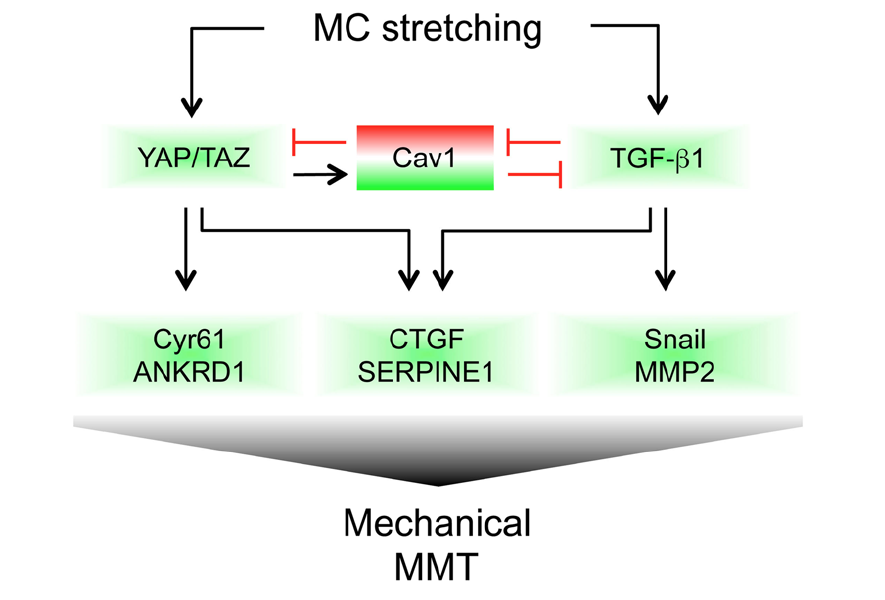
Caveolin1 and YAP drive mechanically induced mesothelial to mesenchymal transition and fibrosis
Raffaele Strippoli, Pilar Sandoval et al. Cell death & disease, 2020.
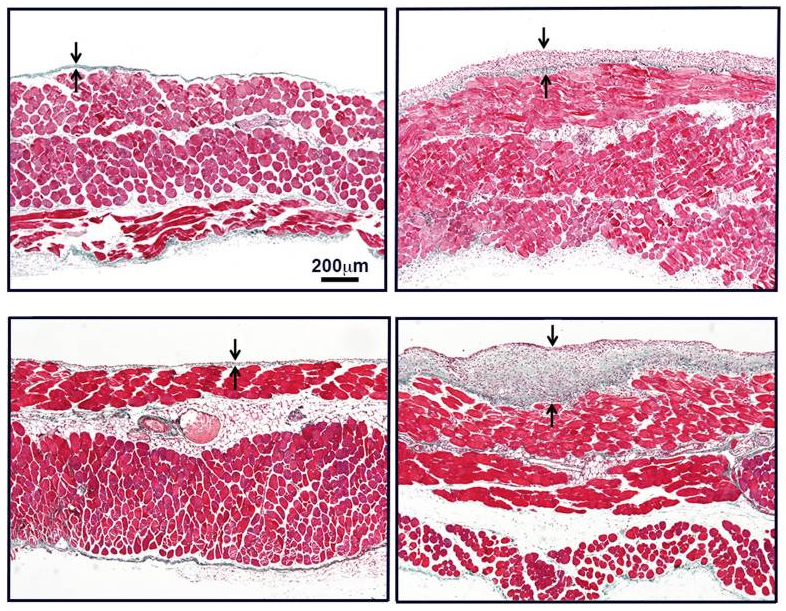
Immune-Regulatory Molecule CD69 Controls Peritoneal Fibrosis
Georgios Liappas et al. J Am Soc Nephrol, 2016.





