Immunoregulatory mechanisms in the development of Chagas disease: translational applications
Research summary:
Chagas disease caused by Trypanosoma cruzi affects approximately 10 million people in Latin America. Besides, blood transfusion and organ transplantation is a sanitary problem in countries receptors of migrants from endemic areas. Cardiac pathology is the most severe and characteristic manifestation and it is estimated a future incidence of between 6.000 y 30.000 cases of chagasic cardiomyopathy in Spain. Our working hypothesis is that the development of pathology depends on a combination of factors: host genetic background, different parasite infecting capacities with different genetic backgrounds, and the regulatory immune response that will affect the inflammatory and the infective processes. Myeloid-derived suppressor cells (MDSC) expand during infection with virulent strains. Figure 1 summarizes the general suppressive mechanisms of MDSC in contact with T cells. On one hand, MDSC expresses nitric oxide synthase (iNOS) and NADPH oxidase 2 (NOX-2) and produces reactive nitrogen species (RNS) and reactive oxygen species (ROS), respectively. Protein nitration and nitrosylation by RNS lead to apoptosis and inhibition of protein phosphorylation and IL-2 mRNA degradation. Arginase 1 (Arg1) together with iNOS consume extracellular L-arginine which inhibits CD3 ζ-chain expression interfering with TCR signaling. Arg1 produces L-ornithine that is the substrate of polyamines linked to proliferation and collagen involved in tissue repair and fibrosis. Cyclooxygenase 2 (COX-2) and microsomal prostaglandin E2 synthase (mPGES-1) produce suppressive prostaglandin E2 (PGE2). Indoleamine 2,3-Dioxygenase (IDO) converts L-tryptophan into L-kynurenine which also blocks T cell proliferation. On the other hand, the SLAMF1 receptor interacting with T. cruzi can trigger the conversion of PI to PI3P activating NOX-2 through the EAT2 adaptor. We observed that nfection by the parasite causes depletion of L-arginine levels, which inhibits T-cell proliferation and RNS including nitric oxide (NO) by iNOS. L-arginine supplementation in infected mice produced a decrease in mortality and an improvement in the clinical score of the mice, which could be used in combined therapy. Furthermore, the immune receptor SLAMF1, regulator of the production of reactive oxygen species (ROS), inhibits ROS production in a strain-dependent manner, so that inhibition of the interaction of SLAMF1 with the parasite could be the basis for a new therapy. Our interest in the translational application of our research leads us maintain scientific collaborations with Spanish and foreign groups, basic and clinical, for the evaluation of new prognostic and follow-up biomarkers, needed for deciding treatment of patients and its efficacy. For this, we use “Omics” tools as Genomics, Transcriptomics, Proteomics, and Metabolomics.
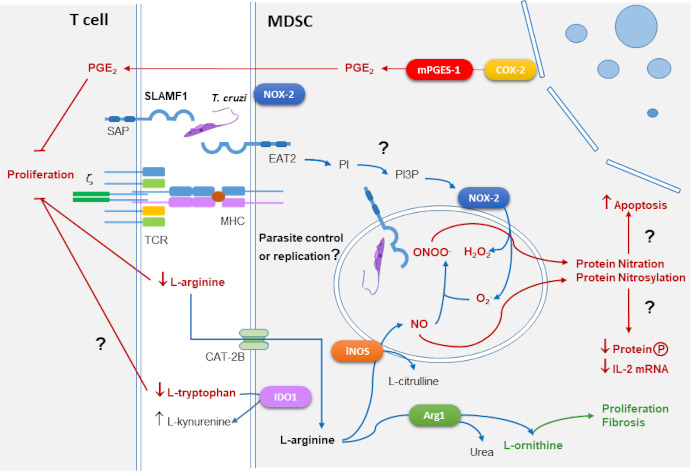
Figure 1. Main mechanisms of T cell immune suppression mediated by MDSCs and SLAMF1 receptor. Question marks indicate those processes that need to be further explored (from Fresno M, Gironès N. 2021. Front Cell Infect Microbiol. Aug 27;11:737364).

| Last name | Name | Laboratory | Ext.* | Professional category | |
|---|---|---|---|---|---|
| Gironés Pujol | Nuria | 226 | 4593/4735 | ngirones(at)cbm.csic.es | Profesor Titular Universidad, GA |
| Santos Peñaloza | Diana Karolina | 226 | 4735 | Tco. de Investigación y Laboratorio |
Relevant publications:
- Fresno M, Gironès N (AC) (2/2). 2021. Myeloid-Derived Suppressor Cells in Trypanosoma cruzi Front Cell Infect Microbiol. Aug 27;11:737364.
- Callejas-Hernández F, Herreros-Cabello A, Del Moral-Salmoral J, Fresno M (AC), Gironès N (AC) (5/5). The Complete Mitochondrial DNA of Trypanosoma cruzi: Maxicircles and Minicircles. Front Cell Infect Microbiol. Jun 29;11:672448.
- Herreros-Cabello A, Callejas-Hernández F, Gironès N, Fresno M (3/4). Trypanosoma cruziGenome: Organization, Multi-Gene Families, Transcription, and Biological Implications. Genes (Basel). Oct 14;11(10):1196.
- Poveda C, Herreros-Cabello A, Callejas-Hernández F, Osuna-Pérez J, Maza MC, Chillón-Marinas C, Calderón J, Stamatakis K, Fresno M, Gironès N. 2020. Interaction of Signaling Lymphocytic Activation Molecule Family 1 (SLAMF1) receptor with Trypanosoma cruzi is strain-dependent and affects NADPH oxidase expression and activity. PLoS Negl Trop Dis. Sep 14;14(9):e0008608.
- Callejas-Hernández F, Gutiérrez-Nogués Á, Rastrojo A, Gironès N, Fresno M. 2019. Analysis of mRNA processing at whole transcriptome level, transcriptomic profile and genome sequence refinement of Trypanosoma cruzi. Sci Rep. Nov 22;9(1):17376.
- Herreros-Cabello A, Callejas-Hernández F, Fresno M, Gironès N. 2019. Comparative proteomic analysis of trypomastigotes from Trypanosoma cruzi strains with different pathogenicity. Infect Genet Evol. Dec;76:104041.
- Callejas-Hernández C, Rastrojo A, Poveda C, Gironès N and Fresno M. 2018. Genomic assemblies of newly sequenced Trypanosoma cruzi strains reveal new genomic expansion and greater complexity. Sci Rep. Oct 2; 8:14631.
- Carbajosa S, Rodríguez-Angulo HO, Gea S, Chillón-Marinas C, Poveda C, Maza MC, Colombet D, Fresno M, Gironès N. L-arginine supplementation reduces mortality and improves disease outcome in mice infected with Trypanosoma cruzi. PLoS Negl Trop Dis. 2018 Jan 16;12(1):e0006179.
- Cuervo H, Guerrero NA, Carbajosa S, Beschin A, De Baetselier P, Gironès N, Fresno M. Myeloid-derived suppressor cells infiltrate the heart in acute Trypanosoma cruzi infection. J Immunol. 2011 Sep 1;187(5):2656-65. doi: 10.4049/jimmunol.1002928. Epub 2011 Jul 29. PMID: 21804013.
- Cuervo H, Pineda MA, Aoki MP, Gea S, Fresno M, Gironès N. Inducible nitric oxide synthase and arginase expression in heart tissue during acute Trypanosoma cruzi infection in mice: arginase I is expressed in infiltrating CD68+ macrophages. J Infect Dis. 2008 Jun 15;197(12):1772-82.
c corresponding author. 1 equal contribution to the direction of the work




















