Physiopathology studies and therapeutical approaches in animal and cellular models of neurometabolic diseases
Research summary:
The group belongs to CIBER Rare Diseases (CIBERER) and to Health Research Institute Hospital La Paz (IdiPAZ) and actively collaborates with Centro de Diagnóstico de Enfermedades Moleculares (CEDEM, Science Faculty, UAM). Our research is focused in neurometabolic diseases, propionic acidemia (PA) and hyperphenylalaninemias (HPAs) among others, enzymatic deficiencies of autosomal recessive inheritance, characterized by the toxic accumulation of precursors and lack of downstream metabolites.
Our projects represent a translational study with the aim of generating and characterizing animal and cellular models relevant for specific diseases, to be used as research tools to understand the molecular and physiopathological mechanisms responsible for disease, to analyse potential biomarkers for prognosis and follow-up and to identify new therapeutical targets. The ultimate aim is to develop personalized RNA targeted therapies (antisense oligonucleotides) as well as pharmacological therapies with antioxidant compounds and mitochondrial activators, performing preclinical studies in the specific disease models.
The research group has ample experience in the in vitro and in vivo use of antisense therapy to revert splicing defects in different rare diseases including HPAs, participating in EU-COST Actions within this topic. To date, we are using gene editing CRISPR/Cas9 technology for the generation of cellular and animal models with specific splicing mutations, to identify and test candidate therapeutical antisense oligonucleotides.
Moreover, we are focused on the analysis of the physiopathological mechanisms underlying PA, one of the most frequent organic acidemias in which we have demonstrated, using a mouse model, that mitochondrial dysfunction, oxidative stress and miRNA dysregulation contribute to the multiorganic complications of the disease. We have revealed alterations in Ca2+ mishandling, associated to elevated ROS levels and higher SERCA2a oxidation rate, along with dysregulation of specific cardiomiRs, as mechanisms involved in the development of PA associated cardiomyopathies. Of note, we have demonstrated the beneficial effect of antioxidants (resveratrol, MitoQ) both in vitro (patients’ fibroblasts) and in vivo (mouse model) and are currently testing other antioxidants and mitochondrial biogenesis activators. We have generated iPS cells derived from patients’ fibroblasts and differentiated them to neurons and cardiomyocytes, relevant cell lineages for the disease, to perform physiopathological studies and testing of pharmacological and genetic therapies.
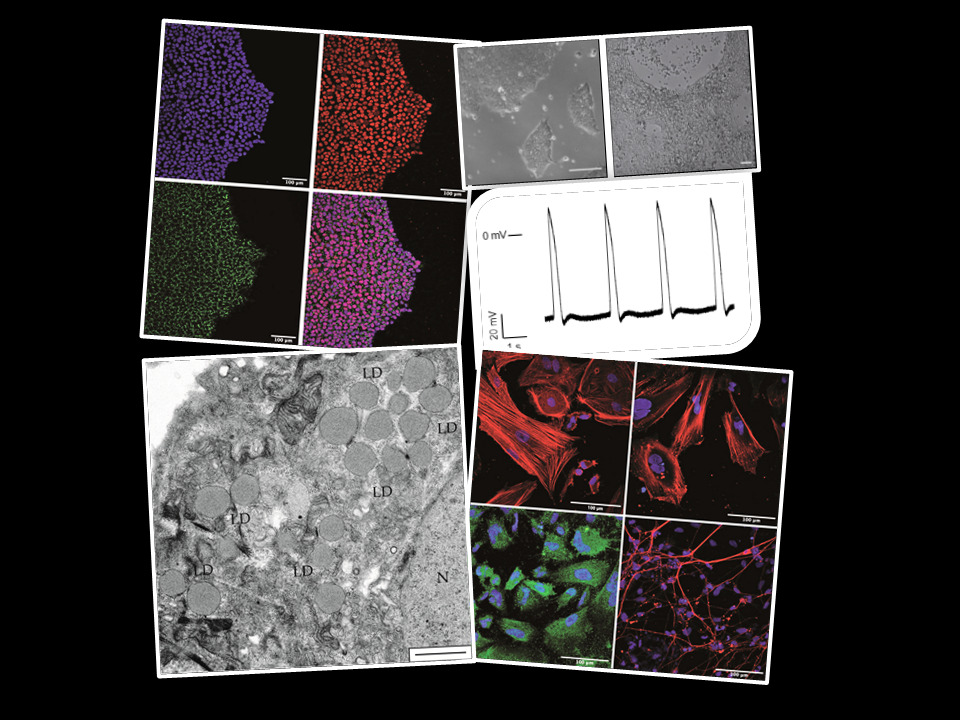
Figure 1. Generation of iPSCs- derived cellular models obtained from propionic acidemia patients´fibroblasts.
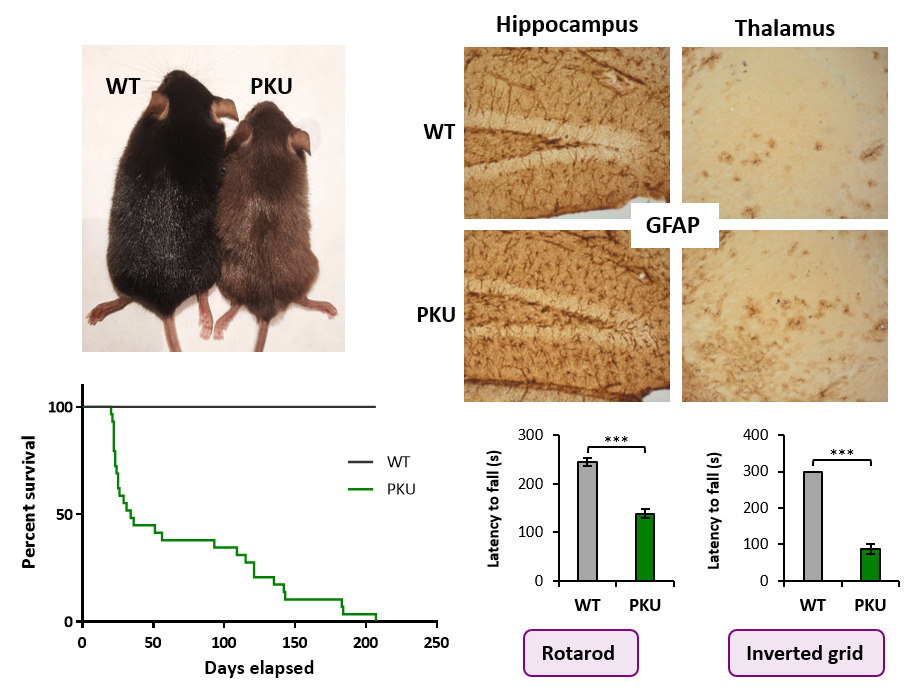
Figure 2. New PKU mouse model generated by CRISPR/Cas9 carrying the frequent splicing variant c.1066-11G>A.

| Last Name | Name | Laboratory | Ext.* | Professional category | |
|---|---|---|---|---|---|
| Alvera Alonso | Beatriz | 220 | 4566 | Estudiante TFG | |
| Álvarez García | María del Mar | 220 | 4596 | malvarez(at)cbm.csic.es | E. Técnicos Superiores Especializados de Organismos Públicos de Investigación |
| González Garnacho | Irene | 220 | 4596 | Estudiante TFG | |
| Martínez Pizarro | Ainhoa | 220 | 4596/7830 | ainhoa.martinez(at)cbm.csic.es | Titulado Sup.de Actividades Técn. y Profes. GP1 |
| Montalvo de la Prida | Elena | 220 | 4596/7830 | emontalvo(at)cbm.csic.es | Tco. de Investigación y Laboratorio |
| Richard Rodríguez | Eva María | 220 | 4628/4596 | erichard(at)cbm.csic.es | Profesor Titular Universidad, GA |
| Ruiz Desviat | Lourdes | 220 | 4566/7830 | lruiz(at)cbm.csic.es | Catedrática |
Relevant publications:
- Martínez-Pizarro A, Leal F, Holm LL, Doktor TK, Petersen USS, Bueno M, Thöny B, Pérez B, Andresen BS, Desviat LR. (2022) Antisense oligonucleotide rescue of deep-intronic variants activating pseudoexons in the 6-Pyruvoyl-Tetrahydropterin Synthase gene. Nucleic Acid Ther. Jul 12. doi: 10.1089/nat.2021.0066
- Soria LR, Makris G, D'Alessio AM, De Angelis A, Boffa I, Pravata VM, Rüfenacht V, Attanasio S, Nusco E, Arena P, Ferenbach AT, Paris D, Cuomo P, Motta A, Nitzahn M, Lipshutz GS, Martínez-Pizarro A, Richard E, Desviat LR, Häberle J, van Aalten DMF, Brunetti-Pierri N. (2022). O-GlcNAcylation enhances CPS1 catalytic efficiency for ammonia and promotes ureagenesis. Nat Commun Sep 5;13(1):5212.
- López Márquez A, Martinez PizarroA, Pérez B, Richard E, Desviat LR. (2022). “Modelling splicing variants amenable to antisense therapy by use of CRISPR-Cas9 based gene editing in HepG2 cells”. Series: Methods Mol Biol. “Antisense RNA design, delivery and analysis”. Arechavala-Gomeza V and Garanto A, eds Springer. Capítulo 10. Pag 167-184. ISSN 1064-3745.
- Alonso-Barroso E, Pérez B, Desviat LR and Richard E. (2021). “Cardiomyocytes derived from induced pluripotent stem cells as a disease model for propionic acidemia”. Int J Mol Sci 22: 1161-1175.
- Hammond SM, Aartsma-Rus A, Alves S, Borgos SE, Buijsen RAM, Collin RWJ, Covello G, Denti MA, Desviat LR, Echevarría L, Foged C, Gaina G, Garanto A, Goyenvalle AT, Guzowska M, Holodnuka I, Jones DR, Krause S, Lehto T, Montolio M, Van Roon-Mom W, Arechavala-Gomeza V. (2021) Delivery of oligonucleotide-based therapeutics: challenges and opportunities. EMBO Mol Med.13(4):e13243.
- Fulgencio-Covián A, Álvarez M, Pepers BA, López-Márquez A, Ugarte M, Pérez B, van Roon-Mom WMC, Desviat LR, Richard E*. (2020). “Generation of a gene-corrected human isogenic line (UAMi006-A) from propionic acidemia patient iPSC with an homozygous mutation in the PCCB gene using CRISPR/Cas9 technology”. Stem Cell Res 49:102055.
- Park KC, Krywawych S, Richard E, Desviat LR, Swietach P. (2020). “Cardiac complications of propionic and other inherited organic acidemias”. Front Cardiovasc Med 7, 1-20.
- Fulgencio-Covián A, Alonso-Barroso E, Guenzel AJ, Rivera-Barahona A, Ugarte M, Pérez B, Barry MA, Pérez-Cerdá C, Richard E, Desviat LR. (2020) Pathogenic implications of dysregulated miRNAs in propionic acidemia related cardiomyopathy. Transl Res. Apr;218:43-56.
- Tamayo M, Fulgencio-Covián A, Navarro-García JA, Val-Blasco A, Ruiz-Hurtado G, Gil-Fernández M, Martín-Nunes L, Lopez JA, Desviat LR, Delgado C, Richard E, Fernández-Velasco M (2020) Intracellular calcium mishandling leads to cardiac dysfunction and ventricular arrhythmias in a mouse model of propionic acidemia Biochim Biophys Acta Mol Basis Dis. Jan 1;1866(1):165586




















