The role of mitochondria in human pathology
Research summary:
Mitochondria play key roles in cellular metabolism, bioenergetics, the execution of cell death and intracellular signaling. Consistent with its prime physiological roles mitochondrial dysfunction is involved in the genesis and progression of ageing and of a plethora of human pathologies including cancer, metabolic syndrome, neurodegeneration and rare disorders. The mitochondrial ATP synthase is a key transducer in energy conservation by oxidative phosphorylation (OXPHOS), in the execution of cell death and in intracellular signaling by reactive oxygen species (ROS). Previously, we have documented the mechanisms and role played by the ATP synthase in metabolic reprogramming during liver development and in human carcinomas. More recently, we have demonstrated that the inhibitor of the ATP synthase, named ATPase Inhibitory Factor 1 (IF1), is highly overexpressed in carcinomas playing a pivotal role in metabolic reprogramming of cancer and stem cells and in liver oncogenesis. We have shown that the binding of IF1 to the ATP synthase inhibits the enzyme under normal physiological conditions and its binding is prevented by phosphorylation of S39 in IF1 by a mitochondrial cAMP-dependent protein kinase A like activity. Inhibition of the ATP synthase by IF1 is required for adaptation to hypoxia, cell cycle progression and in cancer. Contrariwise, dephosphorylation of IF1 is required to increase the mitochondrial output of ATP in response to an increase in energy demand in heart in vivo. Moreover, the IF1-mediated inhibition of the ATP synthase triggers a ROS signal that promotes the activation of nuclear programs of proliferation and resistance to cell death. Hence, IF1 is a most relevant mitochondrial protein that participates in defining the cellular phenotype.
A main objective of our group is to deepen into the cellular biology and role of the ATP synthase/IF1 axis in cancer, neuronal function, metabolic disorders and in ageing. Our research has a clear translational orientation by leading units “713” of the CIBER of Enfermedades Raras (CIBERER) and “Metabolismo Energético Traslacional” of the i+12 of the “Instituto de Salud Carlos III” initiatives. To cover these aims, we have developed transgenic mice (Tg-IF1) that conditionally overexpress human IF1 in neurons, liver, colon (Fig. 1), heart or skeletal muscle, and generated the ATP5IF1 lox/lox mouse which has been successfully used to knock-out IF1 (IF1-KO) in neurons or in enterocytes. With these mouse models we have demonstrated in vivo the role of the ATP synthase/IF1 in metabolic reprogramming and in signaling adaptive cellular and tissue responses in normal and pathophysiological situations (Fig. 2). Besides, we have developed the PROTEOmAb Platform, based on “reverse phase protein array” technologies, to identify new biomarkers of human disorders. These studies are combined with screenings for FDA-approved small molecules that modulate mitochondrial OXPHOS. We anticipate that the combined approach will provide rapid and effective bed-side translation of new drugs for the treatment of pathologies and conditions associated to mitochondrial dysfunction.
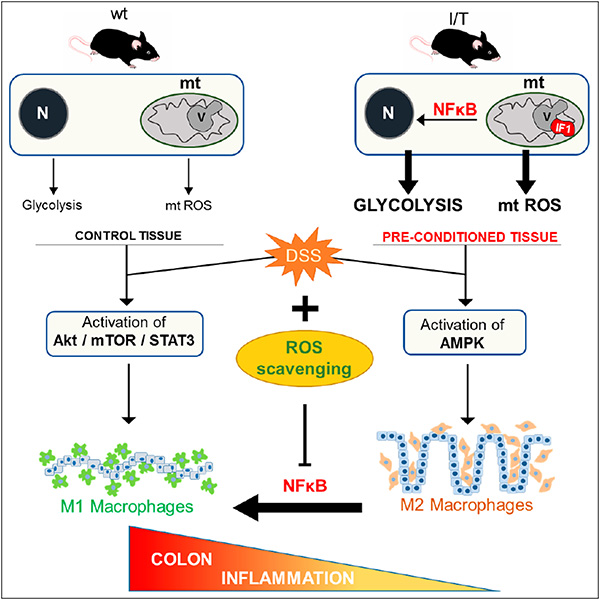
Fig. 1: Role of mitochondrial ROS signaling in defining the immune response of the colon. Graphical abstract taken from reference Cell Rep. 2017 May 9;19(6):1202-1213.
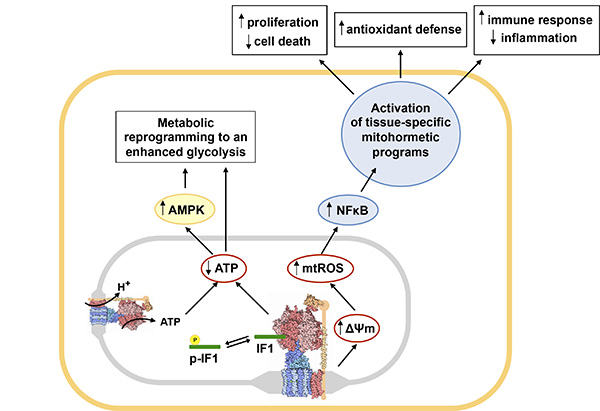
Fig. 2: Main metabolic and redox circuits regulated by the inhibition of the ATP synthase by dephosphorylated IF1. Figure taken from reference Front Oncol. 2018 Mar 7;8:53.

| Last name | Name | Laboratory | Ext.* | Professional category | |
|---|---|---|---|---|---|
| Domínguez Zorita | Sonia | 326 | 4648 | sonia.dominguez(at)cbm.csic.es | Tit.Sup.Activ.Técn.y Profes. GP1 |
Relevant publications:
- González-Llorente L, Santacatterina F, García-Aguilar A, Nuevo-Tapioles C, González-García S, Tirpakova Z, Toribio ML, Cuezva JM. Overexpression of Mitochondrial IF1 Prevents Metastatic Disease of Colorectal Cancer by Enhancing Anoikis and Tumor Infiltration of NK Cells. Cancers (Basel). 2019 Dec 19;12(1). pii: E22. doi: 10.3390/cancers12010022
- Esparza-Moltó PB, Nuevo-Tapioles C, Chamorro M, Nájera L, Torresano L, Santacatterina F, Cuezva JM. Tissue-specific expression and post-transcriptional regulation of the ATPase inhibitory factor 1 (IF1) in human and mouse tissues. FASEB J. 2019 Feb;33(2):1836-1851. doi: 10.1096/fj.201800756R.
- Santacatterina F, Torresano L, Núñez-Salgado A, Esparza-Molto PB, Olive M, Gallardo E, García-Arumi E, Blazquez A, González-Quintana A, Martín MA, Cuezva JM. Different mitochondrial genetic defects exhibit the same protein signature of metabolism in skeletal muscle of PEO and MELAS patients: A role for oxidative stress. Free Radic Biol Med. 2018 Oct;126:235-248. doi: 10.1016/j.freeradbiomed.2018.08.020.
- Formentini L, Santacatterina F, Núñez de Arenas C, Stamatakis K, López-Martínez D, Logan A, Fresno M, Smits R, Murphy MP, Cuezva JM. Mitochondrial ROS Production Protects the Intestine from Inflammation through Functional M2 Macrophage Polarization. Cell Rep. 2017 May 9;19(6):1202-1213. doi: 10.1016/j.celrep.2017.04.036.
- Santacatterina F, Sánchez-Cenizo L, Formentini L, Mobasher MA, Casas E, Rueda CB, Martínez-Reyes I, Núñez de Arenas C, García-Bermúdez J, Zapata JM, Sánchez-Aragó M, Satrústegui J, Valverde ÁM, Cuezva JM. Down-regulation of oxidative phosphorylation in the liver by expression of the ATPase inhibitory factor 1 induces a tumor-promoter metabolic state. Oncotarget. 2016 Jan 5;7(1):490-508. doi: 10.18632/oncotarget.6357.
- García-Bermúdez J, Sánchez-Aragó M, Soldevilla B, Del Arco A, Nuevo-Tapioles C, Cuezva JM. PKA Phosphorylates the ATPase Inhibitory Factor 1 and Inactivates Its Capacity to Bind and Inhibit the Mitochondrial H(+)-ATP Synthase. Cell Rep. 2015 Sep 29;12(12):2143-55. doi: 10.1016/j.celrep.2015.08.052.
- Formentini L, Pereira MP, Sánchez-Cenizo L, Santacatterina F, Lucas JJ, Navarro C, Martínez-Serrano A, Cuezva JM. In vivo inhibition of the mitochondrial H+-ATP synthase in neurons promotes metabolic preconditioning. EMBO J. 2014 Apr 1;33(7):762-78. doi: 10.1002/embj.201386392.
- Sánchez-Aragó M, García-Bermúdez J, Martínez-Reyes I, Santacatterina F, Cuezva JM. Degradation of IF1 controls energy metabolism during osteogenic differentiation of stem cells. EMBO Rep. 2013 Jul;14(7):638-44. doi:10.1038/embor.2013.72.
- Formentini L, Sánchez-Aragó M, Sánchez-Cenizo L, Cuezva JM. The mitochondrial ATPase inhibitory factor 1 triggers a ROS-mediated retrograde prosurvival and proliferative response. Mol Cell. 2012 Mar 30;45(6):731-42. doi:10.1016/j.molcel.2012.01.008.
- Sánchez-Cenizo L, Formentini L, Aldea M, Ortega AD, García-Huerta P, Sánchez-Aragó M, Cuezva JM. Up-regulation of the ATPase inhibitory factor 1 (IF1) of the mitochondrial H+-ATP synthase in human tumors mediates the metabolic shift of cancer cells to a Warburg phenotype. J Biol Chem. 2010 Aug 13;285(33): 25308-13. doi: 10.1074/jbc.M110.146480.
Doctoral theses:
- Imke M. Willers. 2011. "Molecular mechanisms controlling the translation of the mRNA encoding the human catalytic β-subunit of mitochondrial H+-ATP synthase in cancer and development” Universidad Autónoma de Madrid. Directores: José M. Cuezva y Álvaro D. Ortega. Sobresaliente “cum laude”.
- Inmaculada Martínez-Reyes. 2012. "Mecanismos y vías de señalización implicados en la reprogramación metabólica en cáncer de colon”. Universidad Autónoma de Madrid. Director: José M. Cuezva. Sobresaliente “cum laude”.
- Laura Sánchez-Cenizo. 2014. "Función oncogénica del inhibidor de la H+-ATP sintasa, IF1: desarrollo y caracterización de modelos transgénicos condicionales y tejido-específicos”. Universidad Autónoma de Madrid. Director: José M. Cuezva. Sobresaliente “cum laude” Premio de la Real Academia de Doctores a la mejor Tesis Doctoral 2014 en el área de Bioquímica (Premio Juan Abelló Pascual I).
- Javier García Bermúdez. 2015. "Regulación de la expresión y actividad de IF1, el inhibidor fisiológico de la H+-ATP sintasa de la mitocondria” Universidad Autónoma de Madrid. Directores: José M. Cuezva and María Sánchez-Aragó. Sobresaliente “cum laude”. Awarded “Premio Extraordinario”.
- Fulvio Santacatterina. 2016. Metabolismo energético en patología y su traslación a la clínica”. Universidad Autónoma de Madrid. Director: José M. Cuezva. Sobresaliente “cum laude”.
- Cristina Nuevo Tapioles. 2019. “Regulación de la OXPHOS mediada por IF1 y su potencial como diana terapéutica en cáncer”. Universidad Autónoma de Madrid.Directores: José M. Cuezva y Laura Formentini. Sobresaliente “cum laude”.
Patents:
- Method for cancer detection, progression analysis and malign tumor prognosis based on the study of metabolic markers of the cell” European Patent: 03 727 509.6; Canadian Patent 2487176; Japanase Patent: 4235610; United States Patent: 7608403; Date: 2009/08/20.
- IF-1 y mutantes del mismo para su uso como medicamento. ES2 335 373; 24/01/2011.
- Marca PROTEOmAb. 3.055.803. Fecha de publicación: 14/03/2013.
- Procedimiento y kit de diagnóstico diferencial de una enfermedad que cursa con afectación muscular. 10/09.2015.
Other activities:
- We are Unit 713 of CIBERER, in the field of Mitochondrial Pathology of the CIBER de Enfermedades Raras, Instituto de Salud Carlos III.
- We are the Research Group leading “Translation of Energy Metabolism” in the field of Cancer of the Instituto de Investigación Hospital 12 de Octubre (i+12).
- We have coordinated the MITOLAB Consortium of the Comunidad de Madrid.
- Organized de “MITOLAB Closing Meeting” of the Madrid I+D Program, 12-13/11/2015.




















