Research line: Translational Energy Metabolism
Research summary:
- Past achievements
During the past years, our research has been focused on understanding how mitochondrial energy metabolism contributes to the integration of cellular functions, leading to the onset and progression of various pathologies. Complex regulatory mechanisms enable mitochondrial metabolism to meet cellular demands, which go beyond ATP production. We have demonstrated that mitochondrial oxidative phosphorylation also plays additional roles in controlling cell immunity and inflammation (Formentini L. et al., Cell Reports, 2017, PMID: 28494869) and regulating intra- and inter-cellular oncogenic signals (Nuevo-Tapioles, C. et al., Nature Communications, 2020, PMID: 32681016). Impaired mitochondrial function also significantly affects adipose tissue and skeletal muscle lipid species and metabolism (Formentini L et al., Diabetologia, 2017, PMID: 28770317; Sanchez-Gonzalez C et al, EMBO J. 2020, PMID: 32488939). Interestingly, these metabolic disturbances impair ROS and calcium signaling, leading to profound changes in muscle structure (Sanchez-Gonzalez C et al, Cell Death and Disease 2022, PMID: 32488939), thus emerging as key hallmarks of myopathies. Very recently, we have demonstrated the existence of a metabolon in skeletal muscle, aimed at integrating nutrient catabolism with mitochondrial efficiency (Nat Metabolism 2024, doi: 10.1038/s42255-023-00956-y).
- Current Aims
One of the main goals of my research line, supported by PID2022-136738OB-I00 national funding and Fundación Ramón Areces, is to further investigate mitochondrial metabolism in pathophysiology. Using two conditional and tissue-specific mouse models with impaired mitochondrial activity (dysfunctional oxidative phosphorylation mice, LowOXPHOS mice; dysfunctional fatty acid oxidation mice, LowFAO mice), our research group is elucidating how different mitochondrial dysfunctions, environmental factors, and diets impact metabolism at the cellular, tissue, and organismal levels. We aim to identify the aspects of mitochondrial activity that limit cell homeostasis and understand which products of metabolism are essential for proper organism function, as well as how cells obtain or transform them in physiological tissue environments. This knowledge is crucial for exploiting mitochondrial metabolism for therapeutic purposes in the field of cancer and ageing.
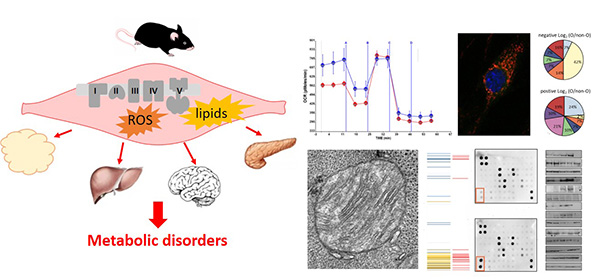
We are defining how skeletal muscle mitochondria dysfunctions act in an autocrine, paracrine and endocrine manner to regulate tissue metabolism. Our final aim is to identify aspects of mitochondria activity that are limiting for whole-body homeostasis in different contexts.

| Last name | Name | Laboratory | Ext.* | Professional category | |
|---|---|---|---|---|---|
| Formentini | Laura | 326 | 4618 | lformentini(at)cbm.csic.es | Profesor Contratado Doctor Universidad, GA |
| Lanza Arnaiz | Jose María | 326 | 4648 | jmlanza(at)cbm.csic.es | Contrato Predoctoral |
| Naharro Naharro | Laura | 326 | 4648 | Estudiante TFG | |
| Salegi Ansa | Beñat | 326 | 4648 | bsalegi(at)cbm.csic.es | Contrato Predoctoral |
| Topdjian Panosian | Tzoline | 326 | 4648 | ttopdjian(at)cbm.csic.es | Tco. de Investigación y Laboratorio |
Relevant publications:
- Juan Cruz Herrero Martín #, Beñat Salegi Ansa #, Gerardo Álvarez-Rivera , Sonia Domínguez-Zorita, Pilar Rodriguez-Pombo, Belén Perez, Enrique Calvo, Alberto Paradela, David G. Miguez, Alejandro Cifuentes, José M. Cuezva, Laura Formentini. An ETFDH-driven metabolon supports OXPHOS efficiency in skeletal muscle by regulating coenzyme Q homeostasis. Nature Metabolism doi: 10.1038/s42255-023-00956-y
- Sánchez-González C, Herrero Martín JC, Salegi Ansa B, Núñez de Arenas C, Stančič B, Pereira MP, Contreras L, Cuezva JM, Formentini L. Chronic inhibition of the mitochondrial ATP synthase in skeletal muscle triggers sarcoplasmic reticulum distress and tubular aggregates. Cell Death & Disease 2022 Jun 22;13(6):561. doi: 10.1038/s41419-022-05016-z. PMID: 35732639; PMCID: PMC9217934.
- Sanchez-Gonzalez C., Nuevo-Tapioles C, Herrero-Martín J, Pereira MP, Cuezva JM, Formentini L. Dysfunctional muscle oxidative phosphorylation shunts BCCA catabolism onto lipogenesis in skeletal muscle. EMBO J. 2020. e103812.
- Nuevo-Tapioles C, Santacatterina F, Stamatakis K, Nuñez de Arenas C, Gomez de Cedron, M, Formentini L and Cuezva JM. Coordinate β-adrenergic inhibition of mitochondrial activity and angiogenesis arrest tumor growth. Nature Communications 11:3606.
- Formentini L*(*: corresponding author), Ryan AJ, Gálvez-Santisteban M, Carter L, Taub P, Lapek JD Jr, Gonzalez DJ, Villarreal F, Ciaraldi TP, Cuezva JM, Henry RR. Mitochondrial H+-ATP synthase in human skeletal muscle: contribution to dyslipidaemia and insulin resistance. Diabetologia. 2017 Oct;60(10):2052-2065.
- Formentini L, Santacatterina F, Núñez de Arenas C, Stamatakis K, López-Martínez D, Logan A, Fresno M, Smits R, Murphy MP, Cuezva JM. Mitochondrial ROS Production Protects the Intestine from Inflammation through Functional M2 Macrophage Polarization. Cell Rep. 2017 May 9;19(6):1202-1213. Impact factor: 2
- Formentini L, Pereira MP, Sánchez-Cenizo L, Santacatterina F, Lucas JJ, Navarro C, Martínez-Serrano A and Cuezva JM. In vivo inhibition of the mitochondrial H+-ATP synthase in neurons promotes metabolic preconditioning. EMBO J. 2014 Apr 1; 33(7):762-78.
- Formentini L, Sanchez Aragó M, Sanchez-Cenizo L, Cuezva J.M. The mitochondrial ATPase Inhibitory Factor 1 (IF1) triggers a ROS-mediated retrograde pro-survival and proliferative response. Mol Cell. 2012 Mar 30;45(6):731-42.
Doctoral theses:
1) Cristina Nuevo Tapioles:
PhD-thesis: “Regulación de la OXPHOS mediada por IF1 y su papel como diana terapéutica en cáncer”.
Thesis Defense: April 26th, 2019, UAM. Sobresaliente “cum laude”.
PhD director: Laura Formentini and J.M Cuezva
2) Cristina Sanchez Gonzalez:
PhD-Thesis: “Papel de la bioenergética mitocondrial sobre el metabolismo del músculo esquelético durante el ejercicio y en patología “.
Thesis Defense: February 8th, 2022, UAM. Sobresaliente “cum laude”.
PhD director: Laura Formentini
3) Juan Cruz Herrero Martín (direction)
PhD-Thesis: “Papel de las deshidrogenasas FAD dependientes en la fisiopatología del musculo esquelético”
Thesis Defense: July 6th, 2022, UAM. Sobresaliente “cum laude”.
PhD director: Laura Formentini




















