Biology of human stem cells in translational neuroscience
Research summary:
The concept of treating diseases with replacement cells in not new; blood transfusions, skin grafts and organ transplantation are all forms of cell replacement therapy. Many neurological diseases, like PD, are the result of cell death or degeneration. The exponentially growing impairment/death rate of dopaminergic neurons (DAn) in the midbrain’s substantia nigra (the A9 subgroup) in PD limits the therapeutic window of the treatments available that are known to increase the quality of life of patients although none can prevent the progression of PD.
Consequently, repairing damaged tissue becomes the goal; when cell loss cannot be prevented, cell replacement holds the key to recovery. Cell replacement therapy for PD is based on the concept that DAn implanted ectopically may functionally restore and maintain the DA levels lost in the disease. Clinical research using human fresh fetal ventral mesencephalic (VM) tissue (hfVM, containing some DAn precursors and many other cell types) provided proof of principle of the therapeutic efficacy of dopaminergic transplants on a long-term basis. However, limitations in hfVM supply, along with the variability of results of different clinical trials and the appearance of graft-induced dyskinesias in some patients, have precluded the implantation of tissue transplantation as a clinical therapy. In this context, research on the basic biology of human stem cells acquires special relevance. Our research group is interested in the basic biology of stem cells and the developmental events leading to maturation of neuronal derivatives of use in the study of the human brain and the development of novel cell-based therapies for neurodegenerative diseases (e.g. Parkinson’s and Alzheimer’s disease).
We have studied the trophic actions of human neural and mesenchymal stem cells in experimental in vivo models of PD focusing on the parallelism between pathological changes occurring in the brain vs. neurological and motor alterations. With a multidisdisciplinary approach, we have worked in the development of the technology for externally controllable bioimplants of therapeutic cells on-demand. These bioimplants consisting in multifunctional leaky optoelectrical fiber for potential neuromodulation and as a cell substrate for application in combined optogenetic stem cell therapy.
With the aim of minimizing the number of laboratory animals used for basic research while increasing the body of knowledge on the biology human neural tissue we have developed a research line devoted to the generation of human cerebral organoids with improved features facilitating patterning studies and useful for improving current preclinical research testing.
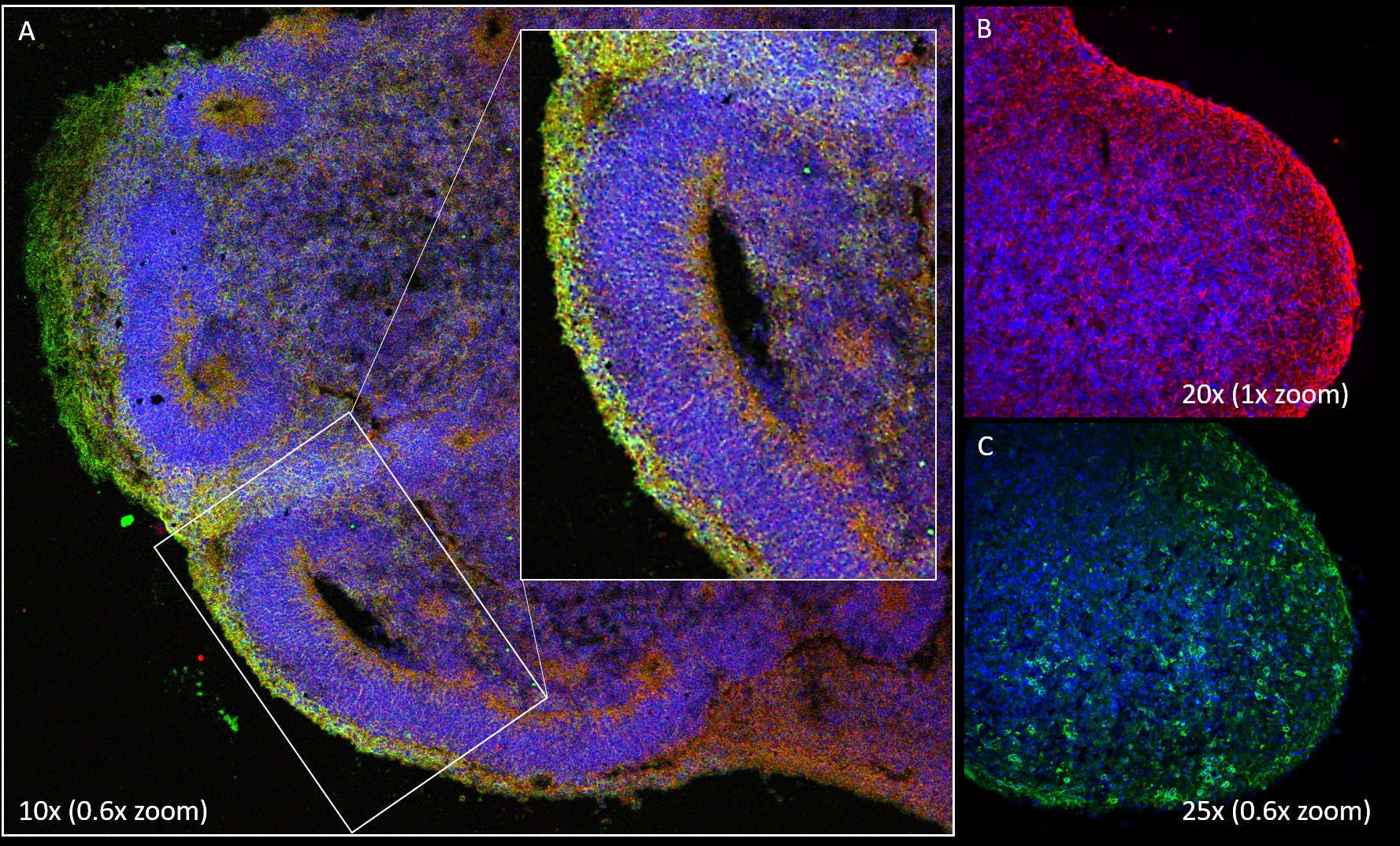
FIGURE 1:
Immunohistochemical staining of a brain organoid (A) and a 3D neural aggregate (B, C).
In the brain organoid, neural stem cells are detected (in red, nestin positive) and immature neurons (in green, beta-III-tubulin positive) with spatial organization after 40 days in culture.
Neural aggregates are formed using human forebrain neural stem cells. After one month in culture, a 3mm long tubular aggregate is formed with neural stem cells expressing nestin (red) and immature neurons (green, beta-III-tubulin). Tissular organization of neural aggregates is in contrast to brain organoid structure.

| Last name | Name | Laboratory | Ext.* | Professional category | |
|---|---|---|---|---|---|
| García Martín | Gonzalo | 305 | 4650 | gonzalo.garcia(at)cbm.csic.es | Titulado Sup.de Actividades Técn. y Profes. GP1 |
| Pérez Pereira | Marta | 305 | 4650 | pereiram(at)cbm.csic.es | Profesor Contratado Doctor Universidad |
| Romero Pérez | Rafael | 305 | 4650 | rromero(at)cbm.csic.es | Titulado Sup.de Actividades Técn. y Profes. GP1 |
| Stancic | Brina | 305 | 4650 | bstancic(at)cbm.csic.es | Titulado Sup.de Actividades Técn. y Profes. GP1 |
| Ulecia Morón | Cristina | 305 | 4650 | cristina.ulecia(at)cbm.csic.es | Titulado Sup.de Actividades Técn. y Profes. GP1 |
Relevant publications:
Vasudevan S, Dotti A, Kajtez J, Martínez-Serrano A, Gundlach C, Maçãs SC, Lauschke K, Vinngaard AM, López SG, Pereira M, Heiskanen A, Keller SS, Emnéus J. Omnidirectional leaky opto-electrical fiber for optogenetic control of neurons in cell replacement therapy. Bioelectrochemistry. 2023 Feb;149:108306. doi: 10.1016/j.bioelechem.2022.108306. Epub 2022 Oct 25. PubMed PMID: 36345111.
Sánchez-González C, Herrero Martín JC, Salegi Ansa B, Núñez de Arenas C, Stančič B, Pereira MP, Contreras L, Cuezva JM, Formentini L. Chronic inhibition of the mitochondrial ATP synthase in skeletal muscle triggers sarcoplasmic reticulum distress and tubular aggregates. Cell Death Dis. 2022 Jun 22;13(6):561. doi: 10.1038/s41419-022-05016-z. PubMed PMID: 35732639; PubMed Central PMCID: PMC9217934.
Nelke A, García-López S, Martínez-Serrano A, Pereira MP. Multifactoriality of Parkinson's Disease as Explored Through Human Neural Stem Cells and Their Transplantation in Middle-Aged Parkinsonian Mice. Front Pharmacol. 2021;12:773925. doi: 10.3389/fphar.2021.773925. eCollection 2021. PubMed PMID: 35126116; PubMed Central PMCID: PMC8807563.
Rothenbücher TSP, Gürbüz H, Pereira MP, Heiskanen A, Emneus J, Martinez-Serrano A. Next generation human brain models: engineered flat brain organoids featuring gyrification. Biofabrication. 2021 Mar 16;13(1):011001. doi: 10.1088/1758-5090/abc95e. PubMed PMID: 33724233.
Alcover-Sanchez B, Garcia-Martin G, Escudero-Ramirez J, Gonzalez-Riano C, Lorenzo P, Gimenez-Cassina A, Formentini L, de la Villa-Polo P, Pereira MP, Wandosell F, Cubelos B. Absence of R-Ras1 and R-Ras2 causes mitochondrial alterations that trigger axonal degeneration in a hypomyelinating disease model. Glia. 2021 Mar;69(3):619-637. doi: 10.1002/glia.23917. Epub 2020 Oct 3. PubMed PMID: 33010069.
Asif A, García-López S, Heiskanen A, Martínez-Serrano A, Keller SS, Pereira MP, Emnéus J. Pyrolytic Carbon Nanograss Enhances Neurogenesis and Dopaminergic Differentiation of Human Midbrain Neural Stem Cells. Adv Healthc Mater. 2020 Oct;9(20):e2001108. doi: 10.1002/adhm.202001108. Epub 2020 Sep 9. PubMed PMID: 32902188.
Sánchez-González C, Nuevo-Tapioles C, Herrero Martín JC, Pereira MP, Serrano Sanz S, Ramírez de Molina A, Cuezva JM, Formentini L. Dysfunctional oxidative phosphorylation shunts branched-chain amino acid catabolism onto lipogenesis in skeletal muscle. EMBO J. 2020 Jul 15;39(14):e103812. doi: 10.15252/embj.2019103812. Epub 2020 Jun 3. PubMed PMID: 32488939; PubMed Central PMCID: PMC7360968.
Martín-Hernández D, Pereira MP, Tendilla-Beltrán H, Madrigal JLM, García-Bueno B, Leza JC, Caso JR. Modulation of Monoaminergic Systems by Antidepressants in the Frontal Cortex of Rats After Chronic Mild Stress Exposure. Mol Neurobiol. 2019 Nov;56(11):7522-7533. doi: 10.1007/s12035-019-1619-x. Epub 2019 May 3. PubMed PMID: 31054078.
Martinez-Serrano A, Pereira MP, Avaliani N, Nelke A, Kokaia M, Ramos-Moreno T. Short-Term Grafting of Human Neural Stem Cells: Electrophysiological Properties and Motor Behavioral Amelioration in Experimental Parkinsons Disease. Cell Transplant. 2016 Dec 13;25(12):2083-2097. doi: 10.3727/096368916X692069. Epub 2016 Jun 17. PubMed PMID: 27324617.
Arac A, Grimbaldeston MA, Nepomuceno AR, Olayiwola O, Pereira MP, Nishiyama Y, Tsykin A, Goodall GJ, Schlecht U, Vogel H, Tsai M, Galli SJ, Bliss TM, Steinberg GK. Evidence that meningeal mast cells can worsen stroke pathology in mice. Am J Pathol. 2014 Sep;184(9):2493-504. doi: 10.1016/j.ajpath.2014.06.003. PubMed PMID: 25134760; PubMed Central PMCID: PMC4188278.
Formentini L, Pereira MP, Sánchez-Cenizo L, Santacatterina F, Lucas JJ, Navarro C, Martínez-Serrano A, Cuezva JM. In vivo inhibition of the mitochondrial H+-ATP synthase in neurons promotes metabolic preconditioning. EMBO J. 2014 Apr 1;33(7):762-78. doi: 10.1002/embj.201386392. Epub 2014 Feb 12. PubMed PMID: 24521670; PubMed Central PMCID: PMC4000092.
Arac A, Brownell SE, Rothbard JB, Chen C, Ko RM, Pereira MP, Albers GW, Steinman L, Steinberg GK. Systemic augmentation of alphaB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation. Proc Natl Acad Sci U S A. 2011 Aug 9;108(32):13287-92. doi: 10.1073/pnas.1107368108. Epub 2011 Jul 26. PubMed PMID: 21828004; PubMed Central PMCID: PMC3156222.
Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, McMillan EL, Schaar BT, Svendsen CN, Bliss TM, Steinberg GK. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain. 2011 Jun;134(Pt 6):1777-89. doi: 10.1093/brain/awr094. PubMed PMID: 21616972; PubMed Central PMCID: PMC3102243.
Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, Shamloo M, Hamilton SA, Jiang K, Huhn S, Palmer TD, Bliss TM, Steinberg GK. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011 Feb;29(2):274-85. doi: 10.1002/stem.584. PubMed PMID: 21732485; PubMed Central PMCID: PMC3524414.
Más en : https://www.ncbi.nlm.nih.gov/myncbi/1f_eokr0RKiQX/bibliography/public/
Doctoral theses:
-
Camille Baumlin (2021) “Effects of a neuroprotective stem cell therapy involving Mesenchymal Stem Cells secreting GDNF in Parkinsonian mice from short to long-term”
-
Theresa SP Rothenbücher (2020) “Engineered brain organoids. Developing an improved and larger human brain model in vitro”
- Anna Nelke (2019) “Differential effects of neural stem cell therapy in adult and middle-aged Parkinsonian mice”




















