Molecular basis of neuronal plasticity
Research summary:
Memories are encoded by long-term changes in synaptic efficiency and connectivity. An in-depth knowledge of the molecular basis of synaptic regulation is fundamental to decipher the mechanisms involved in the formation of memories. Our group studies the cellular and molecular mechanisms that modulate the plasticity of neural networks, with the aim of finding molecular targets and effective strategies to improve cognitive performance. Synaptic activity triggers intracellular calcium (Ca+2) oscillations that locally modulate several signaling pathways. Calmodulin (CaM), a protein that binds calcium, translates these oscillations into intracellular signaling events. Its availability and activity are locally regulated by proteins such as neurogranin (Ng), very abundant in the post-synaptic environment, which sequesters CaM in a Ca+2 and phosphorylation dependent manner. We use several preparations including primary cultures of dissociated neurons to understand the role of Ng in events of synaptic plasticity, such as those associated with hebbian plasticity (Long Term Potentiation -LTP- and Long Term Depression -LTD) and homeostatic plasticity (synaptic scaling). For that we use a combination of biochemical, molecular biology, electrophysiology, advanced microscopy and other imaging techniques. Since Ng levels and cognitive performance are positively correlated in the human brain, we are interested to understand the mechanisms underlying the regulation of Ng transcription and its local translation in dendrites. We propose Ng as a molecular target for strategies designed to prevent, treat or alleviate conditions and pathologies associated to impaired cognitive function. We justify this objective on the following premises. First, Ng function is quite restricted to its action in the brain. Thus, Ng deficiency in mice does not cause apparent anatomical or physiological abnormalities, but severe cognitive impairments. And second, targeting Ng expression to improve cognition is very likely devoid of side-effects, since Ng expression is tightly regulated in space and time (only expressed in the postnatal forebrain) and specifically associated to cognitive performance. In summary, a broader and deeper understanding of the role of Ng and other CaM-sequestering proteins in the mechanisms of neuronal plasticity will contribute to the development of newer therapies to improve the cognitive function and quality of life of aging individuals and patients suffering from neurological diseases.
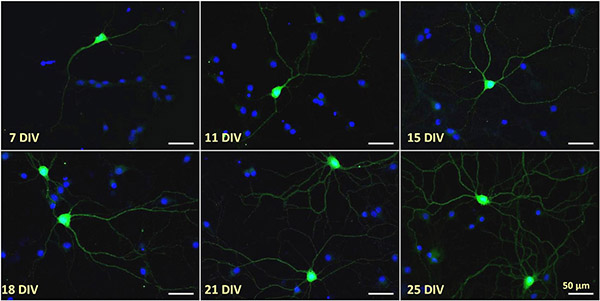
Figure 1.
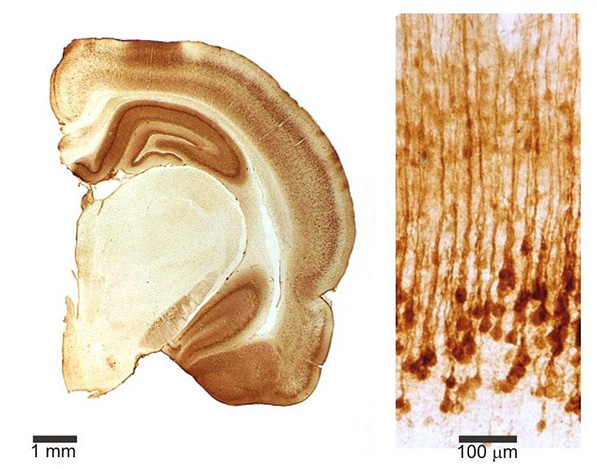
Figure 2.
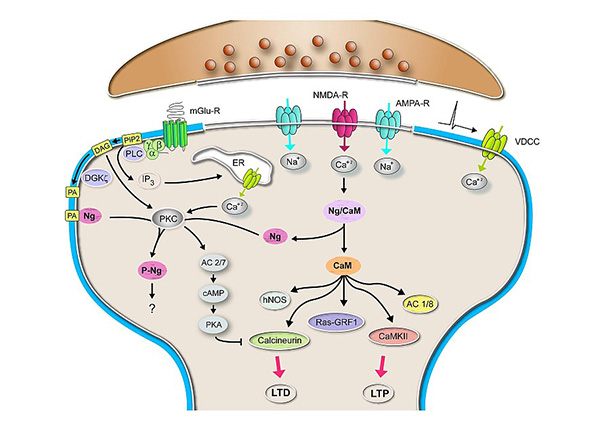
Figure 3.

| Last name | Name | Laboratory | Ext.* | Professional category | |
|---|---|---|---|---|---|
| Díez Guerra | Fco. Javier | 307 | 4612 | fjdiez(at)cbm.csic.es | Catedrático Universidad, GA |
| Martínez Blanco | Elena | 307 | 4642 | elena.martinez(at)cbm.csic.es | Investigador |
Relevant publications:
- Alberto Garrido-García, Raquel de Andrés, Amanda Jiménez-Pompa, Patricia Soriano, Diego Sanz-Fuentes, Elena Martínez-Blanco, F Javier Díez-Guerra. “Neurogranin Expression Is Regulated by Synaptic Activity and Promotes Synaptogenesis in Cultured Hippocampal Neurons”. Molecular Neurobiology 56 (11), 7321-7337 (Nov 2019). DOI: 10.1007/s12035-019-1593-3. PMID 31020616.
- Dolores Piniella, Elena Martínez-Blanco, Ignacio Ibáñez, David Bartolomé-Martín, Eva Porlán, F Javier Díez-Guerra, Cecilio Giménez, Francisco Zafra. “Identification of Novel Regulatory Partners of the Glutamate Transporter GLT-1”. Glia 66 (12), 2737-2755 (Dec 2018). DOI: 10.1002/glia.23524. PMID: 30394597
- Marta Pérez-Hernández, Marcos Matamoros, Silvia Alfayate, Paloma Nieto-Marín, Raquel G Utrilla, David Tinaquero, Raquel de Andrés, Teresa Crespo, Daniela Ponce-Balbuena, B Cicero Willis, Eric N Jiménez-Vázquez, Guadalupe Guerrero-Serna, André M da Rocha, Katherine Campbell, Todd J Herron, F Javier Díez-Guerra, Juan Tamargo, José Jalife, Ricardo Caballero, Eva Delpón. “Brugada Syndrome Trafficking-Defective Nav1.5 Channels Can Trap Cardiac Kir2.1/2.2 Channels”. Journal of Clinical Investigation Insight 2018;3(18):e96291. DOI: 10.1172/jci.insight.96291. PMID: 30232268.
- Daniela Ponce-Balbuena, Guadalupe Guerrero-Serna, Carmen R Valdivia, Ricardo Caballero, F Javier Diez-Guerra, Eric N Jiménez-Vázquez, Rafael J Ramírez, André Monteiro da Rocha, Todd J Herron, Katherine F Campbell, B Cicero Willis, Francisco J Alvarado, Manuel Zarzoso, Kuljeet Kaur, Marta Pérez-Hernández, Marcos Matamoros, Héctor H Valdivia, Eva Delpón, José Jalife. “Cardiac Kir2.1 and Na V 1.5 Channels Traffic Together to the Sarcolemma to Control Excitability”. Circulation Research 122(11):1501-1516 (May 2018). DOI: 10.1161/CIRCRESAHA.117.311872. PMID: 29514831.
- Raquel G Utrilla, Paloma Nieto-Marín, Silvia Alfayate, David Tinaquero, Marcos Matamoros, Marta Pérez-Hernández, Sandra Sacristán, Lorena Ondo, Raquel de Andrés, F Javier Díez-Guerra, Juan Tamargo, Eva Delpón, Ricardo Caballero. “Kir2.1-Nav1.5 Channel Complexes Are Differently Regulated Than Kir2.1 and Nav1.5 Channels Alone”. Frontiers in Physiology 8, 903 (Nov 2017). DOI: 10.3389/fphys.2017.00903. PMID: 29184507.
- Ignacio Ibáñez, F Javier Díez-Guerra, Cecilio Giménez, Francisco Zafra. “Activity Dependent Internalization of the Glutamate Transporter GLT-1 Mediated by β-Arrestin 1 and Ubiquitination”. Neuropharmacology 107, 376-386 (Aug 2016). DOI: 10.1016/j.neuropharm.2016.03.042. PMID: 27044663.
- José P Ferraz-Nogueira, F Javier Díez-Guerra, Juan Llopis. “Visualization of Phosphatidic Acid Fluctuations in the Plasma Membrane of Living Cells”. PLos One 9 (7), e102526 (Jul 2014). DOI: 10.1371/journal.pone.0102526. PMID: 25025521.
- Ana Quintas, Alain J de Solís, F Javier Díez-Guerra, José M Carrascosa, Elena Bogónez. “Age-associated Decrease of SIRT1 Expression in Rat Hippocampus: Prevention by Late Onset Caloric Restriction”. Experimental Gerontology 47 (2), 198-201 (Feb 2012). DOI: 10.1016/j.exger.2011.11.010. PMID: 22143179.
- Carlos Sánchez, Mª Ángeles Muñoz, Maite Villalba, Verónica Labrador, F Javier Díez-Guerra. “Setting Up and Running an Advanced Light Microscopy and Imaging Facility”. Current Protocols Cytometry Chapter 12, Unit 12.22 (Jul 2011). DOI: 10.1002/0471142956.cy1222s57. PMID: 21732308.
- F Javier Díez-Guerra. “Neurogranin, a link between calcium/calmodulin and protein kinase C signaling in synaptic plasticity”. IUBMB Life 62(8):597-606 (2010). DOI: 10.1002/iub.357. PMID: 20665622.
- Alberto Garrido-García, Beatriz Andrés-Pans, Lara Durán-Trío, F Javier Díez-Guerra. “Activity-dependent Translocation of Neurogranin to Neuronal Nuclei”. Biochemical Journal 424 (3), 419-29 (Dec 2009). DOI: 10.1042/BJ20091071. PMID: 19751214.
- Irene Domínguez-González, Silvia N Vázquez-Cuesta, Alicia Algaba, F Javier Díez-Guerra. “Neurogranin Binds to Phosphatidic Acid and Associates to Cellular Membranes”. Biochemical Journal 404 (1), 31-43 (May 2007). DOI: 10.1042/BJ20061483. PMID: 17295609.
- Enrique Fernández-Sánchez, F Javier Díez-Guerra, Beatriz Cubelos, Cecilio Giménez, Francisco Zafra. “Mechanisms of Endoplasmic-Reticulum Export of Glycine transporter-1 (GLYT1)”. Biochemical Journal 409 (3), 669-81 (Feb 2008). DOI: 10.1042/BJ20070533. PMID: 17919119.




















