Morphogenesis and neurodegeneration of the vertebrate Central Nervous System
Research summary:
The brain and other parts of the central nervous system (CNS), such as the retina, develop over a long period of time before reaching their final organization. Such a long period enables millions of neural cells to progressively acquire their specific identity and to progressively establish a precise network of functional connections among the generated cell types. Reaching such an elaborated organization is fundamental for our daily life and well-being. As we age, the brain again undergoes progressive changes but unfortunately “in the reverse direction”, making us less proficient. When these changes are drastic, as it happens in people suffering from Alzheimer’ disease or in individuals who becomes blind because their “photoreceptors” degenerate, performing even simple duties or relating to the immediate surroundings become impossible. Knowing how to have and preserve a healthy brain thus falls in between the comprehension of its development and the identification of the causes of its ageing. Our laboratory divides its research efforts between these two extremes. On one side we investigate a) how the visual system forms; on the other, b) we search the molecular causes underlying the brain alterations occurring in aging and/or Alzheimer ‘disease.
a) For over twenty years, our team has contributed to identify and study the molecular building blocks that coordinate the development of the vertebrate visual system. Building on this experience, we are now investigating how the retinal pigmented epithelium (RPE) forms and contributes to the overall architecture of the eye. The RPE consists of a monolayer of cells, positioned at the back of the neural retina. This tissue has the critical function of protecting, nourishing and cooperating with the light sensing cells of the retina: the photoreceptors. The cells of the RPE are initially neuroepithelial as those of the neural retina but soon transform in a cuboidal/squamous pigmented layer (Figure 1). The events underlying this transformation are still poorly understood. Using the zebrafish and chick embryos as models, we are studying the cellular and molecular aspects of this transformation and are further asking whether this transformation is necessary for the acquisition of the final cup-shape that the vertebrate eye has. In other words, we want to know what happens to the eye if the RPE does not develop correctly. As an extension of this question, we are also asking how the entire visual system adapts to the abnormal development of one of its parts. For example, what happens to the eye if the thalamic visual structures do not form in an appropriate manner?
b) When the brain ages, the number of functional connections among neurons decreases and the brain assume a state of low but chronic “inflammation”. These changes are exacerbated in neurodegenerative diseases such as Alzheimer’s Disease (AD), in which there is also an accumulation of typical “amyloid plaques” (Figure 2). From a molecular point of view, loss of connections, inflammation and amyloid plaque formation have a commonality: the activity of the metalloprotease ADAM10. We have recently shown that a small secreted protein, known as Secreted Frizzled Related Protein 1 (SFRP1), acts as an endogenous inhibitor of ADAM10. Based on this observation, we hypothesized that abnormally upregulated SFRP1 levels in the brain of AD patients could impair ADAM10 activity and be a common trait of AD. Our work has validated this hypothesis, demonstrating that SFRP1 contributes to AD pathogenesis, whereas neutralization of its activity in a mouse model prevents the progression of the disease and the loss of memory present in AD (or in aging). Based on this exciting result and taking advantage of different transgenic mouse models that we have generated, we are now investigating the direct implication of Sfrp1 in the loss of brain connections and in the inflammatory events that take place in AD and aging. We are further exploring the potential of SFRP1 neutralization as a therapeutic strategy against AD.
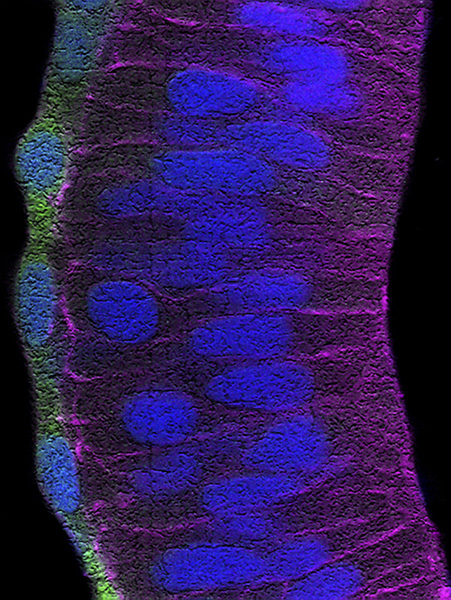
Figure 1: Developing zebrafish retina in which the retinal pigmented epithelium is labelled in green whereas the contour of the neural retina cells is marked in purple. Nuclei of all cells are labelled in blue.
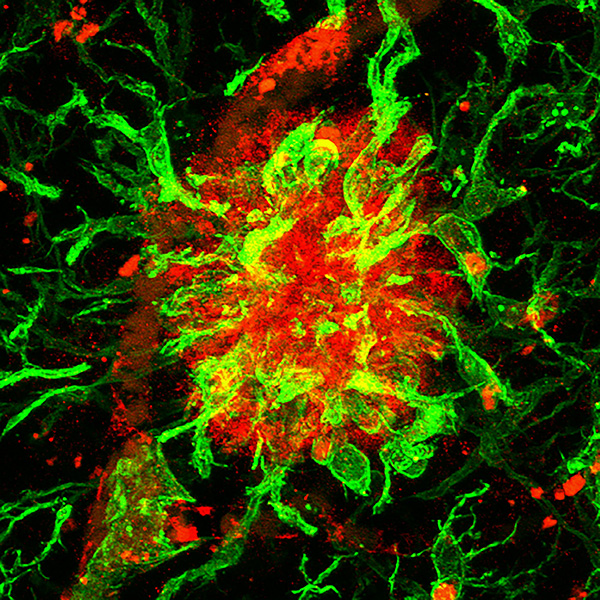
Figure 2: Example of a human amyloid plaque positive for SFRP1 (red) and surrounded by microglial cells (green).

| Last name | Name | Laboratory | Ext.* | Professional category | |
|---|---|---|---|---|---|
| Bovolenta Nicolao | Paula | 426 | 4718 | pbovolenta(at)cbm.csic.es | E. Profesores de Investigación de Organismos Públicos de Investigación |
| Martín Bermejo | Mª Jesús | 426 | 4720 | cbermejo(at)cbm.csic.es | Técnico Sup. Actividades Téc. y Profes. GP3 |
| Martínez Baños | Marcos | 426 | 4720 | mmartinez(at)cbm.csic.es | Titulado Sup. Actividades Tecn. y Prof.GP1 |
| Miaja Hernández | Pablo | 426 | 4720 | pablo.miaja(at)cbm.csic.es | Titulado Sup. Actividades Tecn. y Prof.GP1 |
| Pajda Szeligowska | Eva | 426 | 4720 | eva.pajda(at)cbm.csic.es | M3 Predoc.formación |
| Pereyra Gómez | Guadalupe | 426 | 4720 | gpereyra(at)cbm.csic.es | M3 |
| Pérez García | Vicente | 426 | 4718 | vperez(at)cbm.csic.es | E.Científicos Titulares de Organismos Públicos de Investigación |
| Pérez Ramos | Adrián | 426 | 4720 | adrian.perez(at)cbm.csic.es | Doctor FC3 |
| Sánchez Bustamante | María Elena | 426 | 4720 | elena.sanchez(at)cbm.csic.es | Contratado CIBER |
| Tabanera Anguita | Noemí | 426 | 4720 | ntabanera(at)cbm.csic.es | Titulado Sup. Actividades Tecn.y Prof.GP1 |
Relevant publications:
- SFRP1 modulates astrocyte-to-microglia crosstalk in acute and chronic neuroinflammation. EMBO Rep. 2021 Sep 27:e51696. doi: 10.15252/embr.202051696. Online ahead of print. PMID: 34569685
-
Stretching of the retinal pigment epithelium contributes to zebrafish optic cup morphogenesis. Elife. 2021 Sep 21;10:e63396. doi: 10.7554/eLife.63396. Online ahead of print. PMID: 34545806
- Esteve P, Rueda-Carrasco J, Mateo, I, Martin-Bermejo MJ, Draffin, J, Pereyra, G, Sandonís A, Crespo I, Moreno I, Garcia-Esparcia P, Gomez-Tortosa E, Rabano A, Fortea J, Alcolea D, Lleo A, Heneka MT, Valpuesta, JM, Esteban, JA, Ferrer I, Dominguez M and Bovolenta P (2019) Elevated levels of Secreted-Frizzled-Related-Protein 1 contribute to Alzheimer’s disease pathogenesis. Nat Neurosci. 22, 1258-1268. (Editors’ choice in Sci. Transl. Med, 2019, 11, eaay7697)
- Bertacchi M., Gruart, A., Kaimakis P., Allet C., Serra L., Giacobini P., Delgado-García JM., Bovolenta P., and Studer M. (2019) Mouse Nr2f1 haploinsufficiency unveils new pathological mechanisms of human BBSOA syndrome. EMBO Mol Med 11: e10291 (cover caption article)
- Esteve P.* Crespo I.*, Kaimakis, P., Sandonís A. and Bovolenta P. (2019) Sfrp1 modulates cell-signaling events underlying telencephalic patterning, growth and differentiation. Cereb. Cortex 229, 1059–1074 (* co-authors)
- Moreno-Marmol, T., Cavodeassi, F. and Bovolenta P. (2018). Setting eyes on the retinal pigment epithelium. Front Cell Dev Biol, 6, 145. doi: 10.3389/fcell.2018.00145.
- Marcos S., Nieto-Lopez F., Sandonis A., Di Marco, F., Cardozo M., Esteve, P. and Bovolenta P. (2015) Secreted Frizzled Related Proteins modulate pathfinding and fasciculation of mouse retina ganglion cell axons by direct and indirect mechanisms. J. Neurosci. 35, 4729-4740 (cover caption article; Selected in The Faculty of 1000)
- Marcos, S. González, M., Beccari, L., Carramolino, L., Martin-Bermejo MJ, Amarie O, Mateos-San Martín D., Torroja C, Bogdanovic O, Doohan R., Puk O, Hrabě de Angelis M, Graw J, Gomez-Skarmeta JL, Casares F, Torres M.*, and Bovolenta P.* (2015) Meis1 coordinates a network of genes implicated in eye development and microphthalmia. Development 142, 3009-3020 * co-senior authors
- Cardozo M., Sánchez-Arrones L., Sandonis A., Sánchez-Camacho C., Gestri G., Wilson SW, Guerrero, I. and Bovolenta P. (2014) Cdon acts as a Hedgehog decoy receptor during proximal-distal patterning of the optic vesicle. Nature Comm. 5:4272. (Selected in The Faculty of 1000)
- Sanchez-Arrones, L.*, Nieto-Lopez, F.*, Sanchez-Camacho, C, Carreres MI, Herrera, E, Okada, A Bovolenta, P. (2013) Shh/Boc signaling is required for sustained generation of ipsilateral-projecting ganglion cells in the mouse retina. J. Neurosci. 33, 8596-607 (Featured article) *Equally contributor
- Esteve P., Sandonìs A., Cardozo M., Malapeira J., Ibañez C., Crespo I., Marcos S., Gonzalez-Garcia S., Toribio M.L., Arribas J., Shimono A., Guerrero I. and Bovolenta P. (2011). Sfrps act as negative modulators of ADAM10 to regulate retinal neurogenesis. Nat. Neurosci. 14, 562-569. (Selected in The Faculty of 1000)
- Esteve, P., Sandonìs A., Ibañez C., Shimono A., Guerrero I. and Bovolenta P. (2011) Secreted Frizzled-Related Proteins are required for Wnt/βcatenin signalling activation in the vertebrate optic cup. Development 138, 4179-4184. (Featured article)
Doctoral theses:
- Yap-Taz en la especificación del epitelio pigmentado de vertebrados. Carlos Camacho de la Macorra. Facultad de Ciencias, Universidad Autónoma de Madrid. Co-Dirección: Dr. Marcos Cardozo. En desarrollo.
- Implicacion de Sfrp1 en envejecimiento cerebral. Guadalupe Pereyra. Facultad de Ciencias, Universidad Autónoma de Madrid. Co-Dirección: Dra. Pilar Esteve. En desarrollo.
- Niveles elevados de SFRP1 en un modelo transgénico de raton desencadenan neuroinflamacion y perdida de memoria. Ines Mateos Ruiz. Facultad de Ciencias, Universidad Autónoma de Madrid. Co-Dirección: Dra. Pilar Esteve. Julio 2019. Sobresaliente Cum Laude.
- Morphogenesis of the zebrafish retinal pigment epithelium and its involvement in optic cup formation. Tania Moreno. Marmol Facultad de Ciencias, Universidad Autónoma de Madrid. Co-Dirección: Dra. Florencia Cavodeassi. Junio 2019. Sobresaliente Cum Laude.
- Sfrp1 promotes neuroinflammation through the modulation of ADAM10 proteolytic activity. Javier Rueda. Facultad de Ciencias, Universidad Autónoma de Madrid. Julio 2017, Co-Dirección: Dra. Pilar Esteve. Julio 2017. Sobresaliente Cum Laude.
Patents:
- “Therapeutic target and monoclonal antibodies against it for the diagnosis and treatment of Alzheimer´s Disease”. Inventores: Paola Bovolenta, Pilar Esteve, Javier Rueda Carrasco, Maria Inés Mateo Ruiz, Maria Jesús Martin Bermejo (CSIC); Mercedes Dominguez Rodriguez Inmaculada Moreno Iruela (ISCIII). Ref. EP19382105
Distinctions:
- Member of ERC Scientific Council
- Member of Electo de EMBO
- Research prize “Fundaluce”




















