Functional interactions between tetraspanin CD9 and cell adhesion molecules
Research summary:
Tetraspanins are highly conserved membrane proteins that function as membrane-organizers and control fundamental cell functions, including adhesion, migration and proliferation. The tetraspanin CD9 specifically associates with different adhesion receptors of the immunoglobulin and integrin families in tetraspanin-enriched microdomains (TEMs). Through these interactions, CD9 exerts important regulatory effects on the function of associated adhesion molecules. Our group has reported that CD9 associates directly with the metalloproteinase ADAM17 on the surface of different types of cells, exerting an inhibitory effect on ADAM17 sheddase activity against a variety of its substrates. Interactions of integrin α5β1 with ADAM17 may occur among molecules expressed on the same cell (cis) or on different cells (trans), with the latter reported to support cell-cell adhesion events. Interestingly, the interaction between integrin α5β1 and the disintegrin domain of ADAM17 has been shown to cause the inhibition of both the adhesive capacity of the integrin (i.e. its ability to bind its ligands) as well as of ADAM17 metalloproteinase activity due to steric hindrance leading to decreased accessibility of its catalytic site for the substrate. In contrast, stimuli that promote the dissociation of the α5β1-ADAM17 complex induce the activation of ADAM17 sheddase activity and increase integrin-mediated cell adhesion. Interestingly, the expression of CD9 on the cell surface abrogates integrin α5β1-mediated cell adhesion to its ligands fibronectin and ADAM17-Fc. In situ proximity ligation assays (PLA) and biochemical experiments based on co-immunoprecipitation collectively revealed that the mechanism by which CD9 inhibits α5β1-mediated cell adhesion involves the reinforcement of cis interactions between ADAM17 and α5β1 on the cell surface, which takes place without alteration in α5β1 integrin affinity but is evidenced by changes in the organization of integrin molecules at the plasma membrane.
Exosomes are a type of extracellular vesicles (EVs) of endocytic origin which serve important intercellular communication functions. Exosomes are produced and released by a large variety of donor cells in the organism and contain a specific cargo of lipids, proteins, miRNAs, mRNAs and DNA which, upon their selective binding and/or uptake can modify the phenotype and trigger a functional response in target cells. Tetraspanins are very abundantly expressed on the surface of different types of extracellular vesicles (EVs), including exosomes, and many research groups consider these proteins as the best markers for these EVs. Our group is currently investigating the role of CD9 as a regulator of adhesion molecules and metalloproteinases involved in the binding and/or uptake of exosomes by target cells.
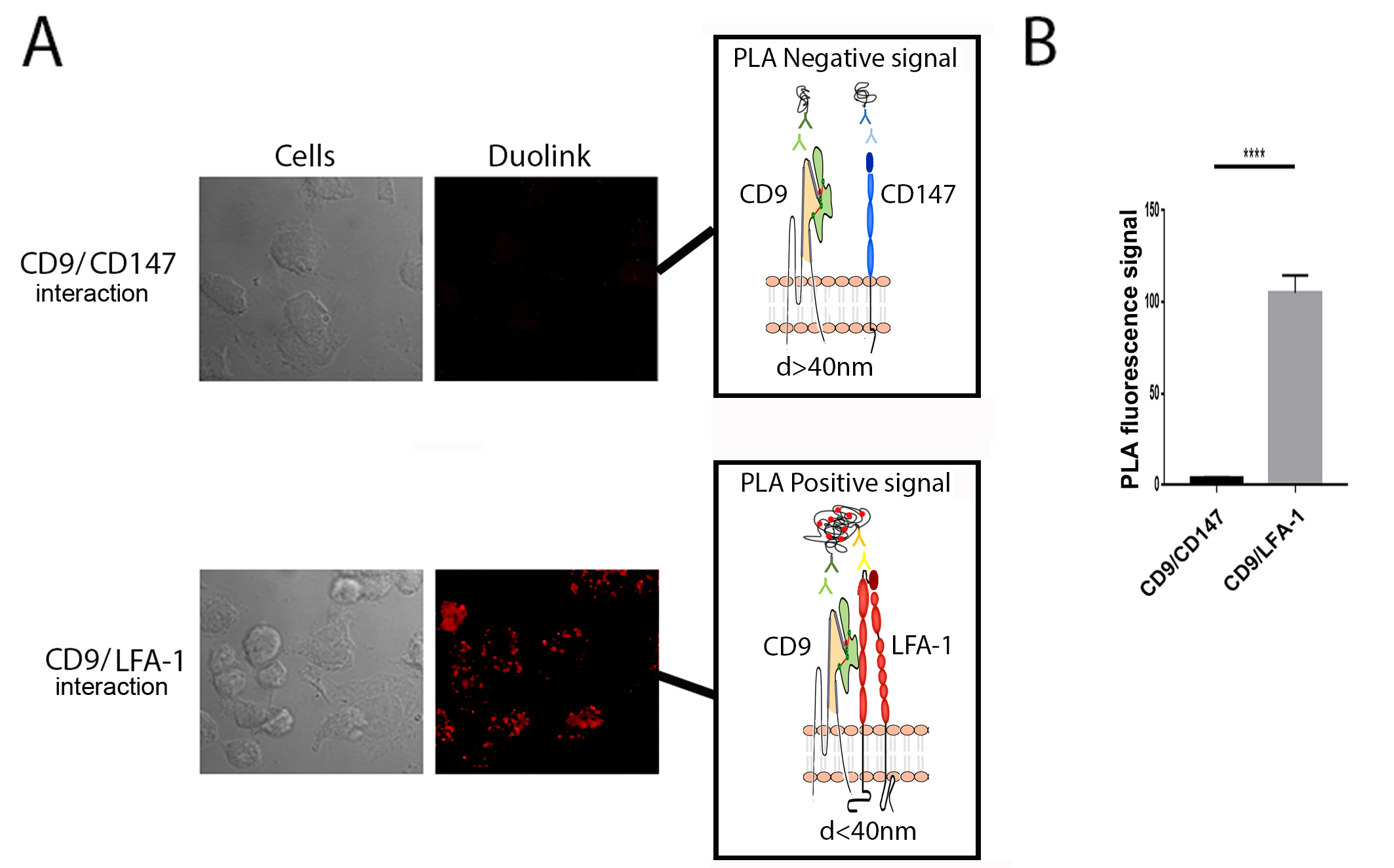
Direct interaction between CD9 and integrin LFA-1 revealed by PLA. (A) Proximity Ligation Assay (PLA) of the molecular association of CD9 with CD147 (negative control) and of CD9 with integrin LFA-1 (positive control) on THP1 macrophage-like cells. (B) The graph represents the quantification PLA signal from panel (A) (mean of PLA/dots from different micrographic fields analyzed by unpaired t test. ****p < 0.0001).

| Last name | Name | Laboratory | Ext.* | Professional category | |
|---|---|---|---|---|---|
| Álvarez Rodríguez | Lara | 125 | 4531 | lalvarez(at)cbm.csic.es | M3 Predoc.formación |
| Cabañas Gutiérrez | Carlos | 125 | 4513 | ccabanas(at)cbm.csic.es | E. Investigadores Científicos de Organismos Públicos |
| Clares Pedrero | Irene | 125 | 4513 | irene.clares(at)cbm.csic.es | M3 Predoc.formación |
| Rocha Mulero | Almudena | 125 | 4513 | arocha(at)cbm.csic.es | M3 Predoc.formación |
Relevant publications:
- Torres-Gómez A, Cardeñes B, Díez-Sainz E, Lafuente EM, & Cabañas C. (2020) Functional Integrin Regulation Through Interactions with Tetraspanin CD9. Methods in Molecular Biology 2217:47-56. (doi: 10.1007/978-1-0716-0962-0_5).
- Torres-Gomez A, Cabañas C, & Lafuente EM. (2020). Phagocytic Integrins: Activation and Signaling. Frontiers in Immunology 11:738. (doi: 10.3389/fimmu.2020.00738).
- Torres-Gomez A, Sanchez-Trincado JL, Toribio V, Torres-Ruiz R, Rodríguez-Perales S, Yáñez-Mó M, Reche PA, Cabañas C, & Lafuente EM. (2020). RIAM-VASP Module Relays Integrin Complement Receptors in Outside-In Signaling Driving Particle Engulfment. Cells 9:1166. (doi: 10.3390/cells9051166).
- Mikuličić S, Fritzen A, Scheffer K, Strunk J, Cabañas C, Sperrhacke M, Reiss K, & Florin L. (2020). Tetraspanin CD9 affects HPV16 infection by modulating ADAM17 activity and the ERK signalling pathway. Medical Microbiology and Immunology 209:461-471. (doi: 10.1007/s00430-020-00671-5).
- Cabañas, C,Yáñez-Mó, M, & van Spriel, A. (2019). Functional relevance of tetraspanins in the immune system. Frontiers in Immunology 10:1714. (doi: 10.3389/fimmu.2019.01714).
- Toribio V, Morales S, López-Martín S, Cardeñes B, Cabañas C, & Yáñez-Mó M. (2019). Development of a quantitative method to measure EV uptake. Scientific Reports 9:10522. (doi: 10.1038/s41598-019-47023-9).
Doctoral theses:
-
Doctorando: Yesenia Machado Pineda (Ftad. Biología, UCM).Título: Caracterización de anticuerpos monoclonales funcionales específicos de ADAM-17Fecha: Noviembre 2017Calificación: Sobresaliente cum laude
-
Doctorando: Raquel Reyes Manzanas (Ftad. Biología, UAM).Título: La tetraspanina CD9 regula la capacidad adhesiva de la integrina leucocitaria LFA-1Fecha: Enero de 2016Calificación: Sobresaliente cum laude
- Iria Medraño Fernández (2012). Papel de la proteína adaptadora RIAM en la fagocitosis mediada por complemento. Universidad Complutense de Madrid. Directores: Dra. Mª Esther Lafuente y Dr. Carlos Cabañas.
- Álvaro Gilsanz Cáceres (2012). La tetraspanina CD9 regula la función de la metaloproteasa ADAM17/TACE y de su sustrato ALCAM. Universidad Complutense de Madrid. Director: Dr. Carlos Cabañas.




















